Assessing Yellow Perch Perca Flavescens Population Dynamics In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Maryland Stream Waders 10 Year Report
MARYLAND STREAM WADERS TEN YEAR (2000-2009) REPORT October 2012 Maryland Stream Waders Ten Year (2000-2009) Report Prepared for: Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 1-877-620-8DNR (x8623) [email protected] Prepared by: Daniel Boward1 Sara Weglein1 Erik W. Leppo2 1 Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 2 Tetra Tech, Inc. Center for Ecological Studies 400 Red Brook Boulevard, Suite 200 Owings Mills, Maryland 21117 October 2012 This page intentionally blank. Foreword This document reports on the firstt en years (2000-2009) of sampling and results for the Maryland Stream Waders (MSW) statewide volunteer stream monitoring program managed by the Maryland Department of Natural Resources’ (DNR) Monitoring and Non-tidal Assessment Division (MANTA). Stream Waders data are intended to supplementt hose collected for the Maryland Biological Stream Survey (MBSS) by DNR and University of Maryland biologists. This report provides an overview oft he Program and summarizes results from the firstt en years of sampling. Acknowledgments We wish to acknowledge, first and foremost, the dedicated volunteers who collected data for this report (Appendix A): Thanks also to the following individuals for helping to make the Program a success. • The DNR Benthic Macroinvertebrate Lab staffof Neal Dziepak, Ellen Friedman, and Kerry Tebbs, for their countless hours in -

2021 Regular Session
HOUSE BILL 843 M2 1lr2482 By: Delegate Howard Introduced and read first time: January 29, 2021 Assigned to: Environment and Transportation A BILL ENTITLED 1 AN ACT concerning 2 Anne Arundel County – Natural Resources – Fishing With a Haul Seine 3 FOR the purpose of repealing certain provisions of law that restrict the length of a haul 4 seine that a person may use in certain waters of Anne Arundel County; repealing a 5 certain penalty for a violation of certain provisions of law that restrict the length of 6 a haul seine that a person may use in certain waters of Anne Arundel County; 7 authorizing a person to use a haul seine to fish in the waters of Anne Arundel County 8 except under certain circumstances; making technical, stylistic, and clarifying 9 changes; and generally relating to fishing with a haul seine in Anne Arundel County. 10 BY repealing and reenacting, without amendments, 11 Article – Natural Resources 12 Section 4–713 13 Annotated Code of Maryland 14 (2018 Replacement Volume and 2020 Supplement) 15 BY repealing and reenacting, with amendments, 16 Article – Natural Resources 17 Section 4–714 18 Annotated Code of Maryland 19 (2018 Replacement Volume and 2020 Supplement) 20 SECTION 1. BE IT ENACTED BY THE GENERAL ASSEMBLY OF MARYLAND, 21 That the Laws of Maryland read as follows: 22 Article – Natural Resources 23 4–713. 24 (a) A person who fishes with haul seine equipment shall possess a tidal fish 25 license to catch finfish. EXPLANATION: CAPITALS INDICATE MATTER ADDED TO EXISTING LAW. [Brackets] indicate matter deleted from existing law. -

Keep Maryland Beautiful Award Recipients
Protecting Land Forever Keep Maryland Beautiful Award Recipients Fiscal Year 2019 Bill James Environmental Grants Historic Sotterley, Inc Howard County Antique Farm Machinery Club Mountain Laurel Garden Club North County High School Pocomoke Middle School Clean Up & Green Up Maryland Grants African American Firefighters Historical Society Alice Ferguson Foundation Allegany County Commissioners & the Allegany County Solid Waste Management Board Annapolis Arts District Annapolis Green, Inc. Antietam-Conococheague Watershed Alliance Back River Restoration Committee, Inc. Banner Neighborhoods Bel Air Downtown Alliance Bethesda Green Beyond the Classroom, Inc. Brunswick Main Street, Inc. BUILD - Rebuild Johnston Square Neighborhood Org C.A.R.E Community Association, Inc Centreville Main Street Town of Centreville City of Greenbelt Department of Public Works Downtown Frederick Partnership Downtown Sykesville Connection at the Community Foundation of Carroll County Druid Heights Community Development Corporation Dundalk Renaissance Corporation Elkton Alliance, Inc. Fusion Partnerships, Inc. (Whitelock Community Farm) Galena Tree and Park Committee Havre de Grace Citizens Against Trash Historic Frostburg - a Maryland Main Street Community Howard County Conservancy I'm Still Standing By Grace Intersection of Change, Inc. Let's Beautify Cumberland! Main Street Historic Chestertown Main Street Middletown, MD Inc Main Street Princess Anne Metropolitan Washington Council of Governments Milton Montford Montgomery Parks Foundation Park Heights Renaissance Pigtown Main Street, Inc. Sandtown South Neighborhood Alliance Southeast Community Development Corporation Strong City Baltimore Takoma/Langley Crossroads Development Authority, Inc. The 6th Branch The Town of Colmar Manor Town of Emmitsburg Town of Manchester Town of Oakland Town of Thurmont & Main Street Westport Community Economic Development Corporation Margaret Rosch Jones Awards All Saints Episcopal Church Cool Green Schools Maryland Coastal Bays Program Sky Valley Association, Inc. -

Watersheds.Pdf
Watershed Code Watershed Name 02130705 Aberdeen Proving Ground 02140205 Anacostia River 02140502 Antietam Creek 02130102 Assawoman Bay 02130703 Atkisson Reservoir 02130101 Atlantic Ocean 02130604 Back Creek 02130901 Back River 02130903 Baltimore Harbor 02130207 Big Annemessex River 02130606 Big Elk Creek 02130803 Bird River 02130902 Bodkin Creek 02130602 Bohemia River 02140104 Breton Bay 02131108 Brighton Dam 02120205 Broad Creek 02130701 Bush River 02130704 Bynum Run 02140207 Cabin John Creek 05020204 Casselman River 02140305 Catoctin Creek 02130106 Chincoteague Bay 02130607 Christina River 02050301 Conewago Creek 02140504 Conococheague Creek 02120204 Conowingo Dam Susq R 02130507 Corsica River 05020203 Deep Creek Lake 02120202 Deer Creek 02130204 Dividing Creek 02140304 Double Pipe Creek 02130501 Eastern Bay 02141002 Evitts Creek 02140511 Fifteen Mile Creek 02130307 Fishing Bay 02130609 Furnace Bay 02141004 Georges Creek 02140107 Gilbert Swamp 02130801 Gunpowder River 02130905 Gwynns Falls 02130401 Honga River 02130103 Isle of Wight Bay 02130904 Jones Falls 02130511 Kent Island Bay 02130504 Kent Narrows 02120201 L Susquehanna River 02130506 Langford Creek 02130907 Liberty Reservoir 02140506 Licking Creek 02130402 Little Choptank 02140505 Little Conococheague 02130605 Little Elk Creek 02130804 Little Gunpowder Falls 02131105 Little Patuxent River 02140509 Little Tonoloway Creek 05020202 Little Youghiogheny R 02130805 Loch Raven Reservoir 02139998 Lower Chesapeake Bay 02130505 Lower Chester River 02130403 Lower Choptank 02130601 Lower -

The State of the Severn River 2018
The State of the Severn River 2018 ITS ALL ABOUT THE TRIBLETS Andrew C. Muller, Ph.D. Diana L. Muller, M.S. Associate Professor Executive Director Oceanography Department Maritimas United States Naval Academy Annapolis, MD. Severn River Association Severn RIVERKEEPER Dr. Muller, Professor of Oceanography, USNA Diana Muller, Executive Director, Maritimas Keith Underwood for his boat Mark Lister for his field work and boat John Page Williams- Chesapeake Bay Foundation And all of the volunteers Methodology Weekly from July to end of October the Severn River Partnership performed vertical water quality monitoring in the tidal estuary using a YSI Pro multi-meter for dissolved oxygen, pH, temperature, salinity, and water samples for bacteria. Dr. Andrew Muller and Capt. Diana Muller trained the Severn River Partnership volunteers by strict QA/QC guidelines as per EPA Chesapeake Bay program methodology and the Maryland Tributary Assessment Coalition (EcoCheck, 2011). The statistical analysis was performed by Dr. Muller and Capt. Muller by MTAC protocols and standard statistical analysis. Statistical analysis was performed using Sigma Plot 12. ArcGIS Pro was utilized to process Landsat 8 and 5 LIDAR data, Google Earth Pro was used to display urban and shoreline changes over time (USGS, 2018) (Globe, 2018). Nutrient data was provided by the Maryland Department of Natural Resources. Water Quality Grades Water Clarity 80% Dissolved Oxygen 62% Bacteria 82% Total Nitrogen 17% Total Phosphorus 50% Dissolved Oxygen The healthy level for dissolved oxygen is 5 mg/L or greater for the Severn River Severe hypoxia occurs at < 2 mg/L and anoxic conditions are when oxygen is below 0.2 mg/L. -
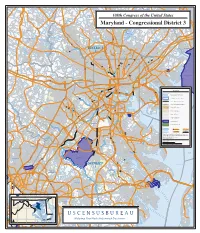
U N S U U S E U R a C S
) d R y r o e ) m k E P d ( Fallston R d ) e l 1 r d o e O 9 g ( R y im S lt id 4 n w a 5 o t B R 8 t H tH ( y g w S S 7 in y tHwy weet Air R w 145 (S 2 h 1 d ( Belair Byp ) B H s 4 d t a 0 a StHwy 165 tR S S ( l S S d W B DISTRICT t w H a t l H DISTRICT i w ti n y m w ) M ) 2 o y e 4 d r 6 k il e 3 l 1 P R ) R B 0 d 108th45 Congress of the United States 1 e d n l y l v ( w R H l ) o d H t i t S R ) r Mill d) v g a r e e s l p n n t a t i P t o ( e h u v r s B r e ( a a r 8 J W R 2 ( d 1 w 6 S ) e y 4 tH w N 1 ( w H t y y 7 S w 1 9 6 H y t 5 Atkisson Reservoir w S ( d F H R t o ir r Aberdeen Proving Ground Edgewood Arsenal S Worthington Rd k A l R e d B Pleasant ) Hills r Rd utle StHwy 91 (Gamber Rd) B S t M Hw y a i 3 n 2 S ( W t a s h in StHwy 152 g Cockeysville CARROLL t o G n r e R e n d s ) p r in g Reisterstown Rd A v Reisterstown G e a r r i s S o tH n w y F 1 S o Loch Raven 2 t r 9 H Reservoir e ( n s P i w l t a r DISTRICT y k N R k DISTRICT n ico d d 4 ra em us R d H F e 5 d ig lv 1 ( h Edgewood B Y 1 t DISTRICT h R d s o Kingsville 2 c r r A Lutherville- u 83 k h v C R e) Mays Ln d Timonium 6 s ) an Owings ym Chapel S rr t Be H w Mills Semi ve DISTRICT y Gre nary A ens 1 p 5 S ring StH w 2 tH Valley Rd y 1 Rd 3 ( w Thornton 1 Hampton 1 y J ) M 1 o d 4 p a p R 795 0 a g ( R d Joppatowne n R d r e o o i t f s r l F i te S a r a a s s l R t l s H o e l ( d r w R ) a n d 7 R h W Joppa Rd d 4 ) C 1 Lakeside y Perry Hall D Blvd w Liberty e H e n t Reservoir r d u R S P P R e d Carney i a ld R Dulaney Valley Rd HARFORD n r e New Town -

Distribution and Abundance of Submerged Aquatic Vegetation in the Chesapeake Bay: a Scientific Summary
W&M ScholarWorks Reports 1-1-1982 Distribution and Abundance of Submerged Aquatic Vegetation in the Chesapeake Bay: A Scientific Summary Robert J. Orth Virginia Institute of Marine Science Kenneth A. Moore Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Marine Biology Commons Recommended Citation Orth, R. J., & Moore, K. A. (1982) Distribution and Abundance of Submerged Aquatic Vegetation in the Chesapeake Bay: A Scientific Summary. Special Reports in Applied Marine Science and Ocean Engineering (SRAMSOE) No. 259. Virginia Institute of Marine Science, College of William and Mary. https://doi.org/10.21220/V58454 This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. DISTRIBUTION AND ABUNDANCE OF SUBMERGED AQUATIC VEGETATION IN THE CHESAPEAKE BAY: A SCIENTIFIC SUMMARY by Robert J. Orth and Kenneth A. Moore Virginia Institute of Marine Science of the College of William and Mary Gloucester Point, Virginia 23062 Special Report No. 259 in Applied Marine Science and Ocean Engineering DISTRIBUTION AND ABUNDANCE OF SUBMERGED AQUATIC VEGETATION IN THE CHESAPEAKE BAY: A SCIENTIFIC SUMMARY by Robert J. Orth and Kenneth A. Moore Virginia Institute of Marine Science of the College of William and Mary Gloucester Point, Virginia 23062 Special Report No. 259 in Applied Marine Science and Ocean Engineering CONTENTS List of Figures •• . iii List of Tables. iv 1. Introduction. 1 2. Methods. 4 3. Present Distribution •• 5 4. -

Severn River Commission Presentation
69 Franklin Street Annapolis, MD 21401 410-216-9441 www.chesapeakelegal.org [email protected] OUR MISSION To use the law to protect and restore the Chesapeake Bay and its watershed by providing pro bono legal assistance through a network of volunteer attorneys. OUR VISION A clean, healthy Chesapeake Bay that can be enjoyed safely by humans, sustain viable fishing harvests, and support thriving and diverse aquatic and wildlife populations. HOW IT WORKS Intake and Evaluation Client CLA Attorney request for assistance connect with assistance Restoration and Protection Our Clients and Partners v Individuals v Ad-Hoc Coalitions v Community Groups v State and Local v Environmental Organizations Government v Conservation Organizations v Home Owners Associations v Coalitions CLA Clients 1000 Friends of Maryland Bedford County Citizen Choose Clean Water Federal Hill Residents Action Committee for Transit Group Coalition Food and Water Watch Agricultural Certainty Work Beechwood Hill Homeowners Choptank Riverkeeper Frederick County Citizens Group Association Citizens Against Plan for Group Allentown Residents for Berrywood Community Eva Mar Development Friends of Bryan Park Clean Air Association Citizens for a Better Charles Friends of Cabin John Creek Alliance for the Chesapeake Blue Water County Watershed Bay Baltimore/Baltimore Citizens to Protect Blu Point Friends of Hillmead Park Alliance to Restore Northwest Harbor Waterkeeper Citizens to Save Little Pimmit Friends of Jug Bay Creek BMAT Restoration Work Run Friends of Mc Millan Park -
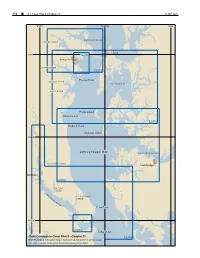
CPB3 C13 WEB.Pdf
316 ¢ U.S. Coast Pilot 3, Chapter 13 19 SEP 2021 76°40'W 76°20'W 76°W MAGOTHY RIVER SEVERN RIVER Kent Island 39°N 12283 Annapolis SOUTH RIVER 12282 omas Point RHODE RIVER EASTERN BAY WEST RIVER Poplar Island HERRING BAY 12270 Holland Point Tilghman Island 38°40'N CHESAPEAKE BAY CHOPTANK RIVER HUNTING CREEK Cambridge Benedict 12266 BATTLE CREEK P A T U ST. LEONARD X E N CREEK T R IV ER Cove Point 38°20'N 12284 Cedar Point Chart Coverage in Coast Pilot 3—Chapter 13 12264 NOAA’s Online Interactive Chart Catalog has complete chart coverage http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 19 SEP 2021 U.S. Coast Pilot 3, Chapter 13 ¢ 317 Chesapeake Bay, Patuxent and Severn Rivers (1) This chapter describes the western shore of Cedar Point. (See 33 CFR 334.200, chapter 2, for limits Chesapeake Bay from Point Lookout, on the north side and regulations.) The target areas in the danger zone are of the entrance to Potomac River, to Mountain Point, marked by lighted buoys. the northern entrance point to Magothy River. Also (12) A middle ground with depths of 10 to 18 feet is about described are Patuxent River, Herring Bay, West River, 8 miles eastward of Point Lookout; the area is about 7 South River, Severn River, and Magothy River, the miles long in a north-south direction and 2 miles wide. bay’s principal tributaries; the ports of Solomons Island, The stranded wreck near the middle of the shoal is marked Benedict, Chesapeake Beach, Shady Side, Galesville and a light. -

Baltimore Region Transportation Improvement Program 2020-2023
BALTIMORE REGION TRANSPORTATION IMPROVEMENT PROGRAM 2020-2023 DEVELOPED BY THE BALTIMORE METROPOLITAN PLANNING ORGANIZATION CITY OF ANNAPOLIS ANNE ARUNDEL COUNTY BALTIMORE CITY BALTIMORE COUNTY CARROLL COUNTY HARFORD COUNTY HOWARD COUNTY QUEEN ANNE’S COUNTY HARFORD TRANSIT MD DEPARTMENT OF TRANSPORTATION MD DEPARTMENT OF THE ENVIRONMENT MD DEPARTMENT OF PLANNING MD TRANSIT ADMINISTRATION The preparation of this document has been financed through funds provided by the Maryland Department of Transportation and the Baltimore Metropolitan Council as matching shares for funds from the Federal Highway Administration and the Federal Transit Administration of the United States Department of Transportation. Electronic copies are available from the: Baltimore Metropolitan Council’s website https://www.baltometro.org/sites/default/files/bmc_documents/general/transportation/tip/20-23/20-23TIP.pdf Print copies are available from the: Baltimore Metropolitan Council 1500 Whetstone Way, Suite 300 Baltimore, Maryland 21230 Phone: 410-732-0500 Fax: 410-732-8248 Transportation Improvement Program - FY 2020-2023 Table of Contents Information about the TIP 1 I.A Introduction - Summary 1 I.B Introduction - Metropolitan Planning Organization Self-Certification 6 I.C Introduction - Consistency with Maximize2045 7 II.A Federal Requirements - Requirements of the Fixing America's Surface Transportation (FAST) Act 9 II.B Federal Requirements - Environmental Justice 12 II.C Federal Requirements - Coordinating Human Service Transportation 20 II.D Federal Requirements - Additional Programs for Seniors and Persons with Disabilities 21 II.E Federal Requirements - Status of Projects from the 2019-2022 TIP and New Projects in the 2020-2023 TIP 23 II.F Federal Requirements - Conformity with Air Quality Planning 35 II.G Federal Requirements - Performance Based Planning and Programming 37 III.A Program Development - Integration with Federal, State and Local Programs 49 III.B Program Development - Federal Fund Sources for Surface Transportation Projects 50 IV. -

Federal Register/Vol. 86, No. 82/Friday, April 30, 2021/Proposed
Federal Register / Vol. 86, No. 82 / Friday, April 30, 2021 / Proposed Rules 22913 DEPARTMENT OF HOMELAND for fireworks displays that take place event, COTP contact information, and SECURITY either on or over the navigable waters of finally the tables of events. the Fifth Coast Guard District as defined As noted above we are proposing to Coast Guard at 33 CFR 3.25. These regulations were add a definition section up front so that last amended June 13, 2017 (81 FR terms utilized later in the regulation are 33 CFR Part 165 81005). The Fifth Coast Guard District is explained to the reader up front. One of the defined terms is ‘‘Event Patrol [Docket Number USCG–2020–0138] proposing to revise these regulations to update existing events, add new events, Commander’’, or ‘‘Event PATCOM’’. RIN 1625–AA00 and remove events that no longer The Event PATCOM replaces the require additional safety measures. current regulation’s reference to the Safety Zones; Recurring Marine Events Based on the nature of fireworks and the ‘‘Coast Guard Patrol Commander’’; there and Fireworks Displays Within the large number of people attending, the is no change to the associated Fifth Coast Guard District Coast Guard has determined that the definition. Use of an Event PATCOM AGENCY: Coast Guard, DHS. events listed in this rule could pose a enables the local Captain of the Port to retain operational control and ACTION: Notice of proposed rulemaking. risk to the public. We are also proposing to revise the text for better clarity and incorporate risk-based decision making SUMMARY: The Coast Guard proposes to easier readability. -

Severn River Summary Report
SEVERN RIVER Watershed Management Master Plan Final Report Submitted to: Anne Arundel County Office of Environmental and Cultural Resources Watershed Management Program 2662 Riva Road Annapolis, MD 21401-7374 Submitted by: KCI Technologies, Inc. 10 North Park Drive Hunt Valley, MD 21030 In Association With: CH2M Hill 13921 Park Center Road, Suite 600 Herndon, VA 20171 February 2006 ACKNOWLEDGEMENTS The Severn River Watershed Management Plan was prepared by staff from KCI Technologies and CH2M HILL with review from Anne Arundel County personnel. Funding and support was provided by Anne Arundel County. The principal authors were: Bill Frost, PE Project Manager, KCI Tara Ajello, PE Project Manager, CH2M HILL Mike Pieper Senior Environmental Scientist, KCI, Support was provided by: Bill Medina Project Engineer, KCI Nate Drescher GIS Specialist, KCI Andy Hu GIS Programmer, CH2M HILL Mark Kramer GIS Programmer, CH2M HILL Lindsay Nicoll Environmental Scientist, KCI Andrea Poling GIS Specialist, KCI Mike Miller Environmental Scientist, KCI Internal review of the report was provided by: Laurens van der Tak, PE Senior Engineer, CH2M HILL The authors would like to thank the following Anne Arundel County staff for their review, comments and advice through the course of the project: Charlie Abrahamson, Office of Planning and Zoning Ronald Bowen, Department of Public Works Ginger Ellis, Office of Environmental and Cultural Resources Ron Etzel, Department of Public Works (retired) Rick Fisher, Office of Environmental and Cultural Resources Hala Flores,