Barstowite, 3Pbcb.Pbc03.H20, a New Mineral from Bounds Cliff, St Endellion, Cornwall
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Billing Outline First Son John Who Married Margery Blewet and Settled at St Tudy in the 1540S
THE HERALD’S VISITATION OF 1620 FOCUSED SOLELY ON THE LINE OF JOHN BILLING / TRELAWDER’S 6 miles BILLING OUTLINE FIRST SON JOHN WHO MARRIED MARGERY BLEWET AND SETTLED AT ST TUDY IN THE 1540S. Summary of what is a rather large chart: BILLING update, December 2018. The rest of the family successfully finished their 1000 National Archives document R/5832 has a supposed date of 24 April 1512; but is This outline sets out the BILLING alias TRELAWDER family connections in Cornwall THIS LINE IS SHOWN HERE IN PURPLE ON THE LEFT HAND SIDE AS SET OUT IN 1874 BY THE HARLEIAN piece jigsaw puzzle; but sadly we have not been so successful in joining together the many over two hundred years. It is unusual to see an alias - our modern equivalent being the SOCIETY AND USED BY SIR JOHN MACLEAN IN HIS RESEARCH. endorsed with a note by C.G.. Henderson “This deed was forged about 17 Eliz. [1577] hundreds of pieces that make up the BILLING alias TRELAWDER story. by Nicholas Beauchamp of Chiton (denounced by the Devon Jury)” hyphenated name - being sustained over so long a time. OTHER BRANCHES OF THE FAMILY STAYED IN ST MINVER AND IN THE ST BREOCK / EGLOSHAYLE AREA. ST TUDY LINE LEFT In many cases, no connections are attempted. At other times links have been suggested. THESE WERE NOT CHRONICLED, BUT WE MAY ASSUME THAT RICHARD, AT ST MINVER IN 1523, AND As mentioned earlier, the 1874 book on the Cornwall Visitations by the Harleian Society, The spelling of TRELAWDER does vary, sometimes TRELODER or TRELOTHER etc. -
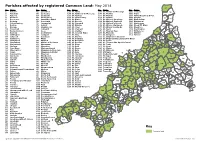
Parish Boundaries
Parishes affected by registered Common Land: May 2014 94 No. Name No. Name No. Name No. Name No. Name 1 Advent 65 Lansall os 129 St. Allen 169 St. Martin-in-Meneage 201 Trewen 54 2 A ltarnun 66 Lanteglos 130 St. Anthony-in-Meneage 170 St. Mellion 202 Truro 3 Antony 67 Launce lls 131 St. Austell 171 St. Merryn 203 Tywardreath and Par 4 Blisland 68 Launceston 132 St. Austell Bay 172 St. Mewan 204 Veryan 11 67 5 Boconnoc 69 Lawhitton Rural 133 St. Blaise 173 St. M ichael Caerhays 205 Wadebridge 6 Bodmi n 70 Lesnewth 134 St. Breock 174 St. Michael Penkevil 206 Warbstow 7 Botusfleming 71 Lewannick 135 St. Breward 175 St. Michael's Mount 207 Warleggan 84 8 Boyton 72 Lezant 136 St. Buryan 176 St. Minver Highlands 208 Week St. Mary 9 Breage 73 Linkinhorne 137 St. C leer 177 St. Minver Lowlands 209 Wendron 115 10 Broadoak 74 Liskeard 138 St. Clement 178 St. Neot 210 Werrington 211 208 100 11 Bude-Stratton 75 Looe 139 St. Clether 179 St. Newlyn East 211 Whitstone 151 12 Budock 76 Lostwithiel 140 St. Columb Major 180 St. Pinnock 212 Withiel 51 13 Callington 77 Ludgvan 141 St. Day 181 St. Sampson 213 Zennor 14 Ca lstock 78 Luxul yan 142 St. Dennis 182 St. Stephen-in-Brannel 160 101 8 206 99 15 Camborne 79 Mabe 143 St. Dominic 183 St. Stephens By Launceston Rural 70 196 16 Camel ford 80 Madron 144 St. Endellion 184 St. Teath 199 210 197 198 17 Card inham 81 Maker-wi th-Rame 145 St. -

Roy Speakman
The Port Isaac, Port Gaverne and Trelights Newsletter No: 232 • June 2003 • 35p of cut grass and blackthorn also provide a warm place for slow- Port Gaverne Quay open again worms and insects. embers of Friends of the Main spent Saturday May 17th working on the If you would like to help and/or join M Quay at Port Gaverne and the good news is that it’s now open again. please contact: Tony Wainwright at Previously, following a major rock fall and the ravages of the sea, stonework [email protected] or was made good and strengthened or replaced to the face of the Quay. All 01208 880846 that was needed to complete the job was to backfill the hole with concrete. or Chris Bolton So almost four tons was mixed on the beach and hoisted up to the Quay - by [email protected] or bucket! Needless to say, everyone slept well that night - and in certain cases 01208 880256 many new muscles were discovered! The next big task is the replacement of the bridge and planning is well advanced. The building team have made all the measurements and got their hard hats ready. FOTM have also received generous donations of materials so work will be starting soon. A photographic record of all the work can be seen on the Friends of the Main website, www.friendsofthemain.org.uk including a picture of a sea-going trailer - quite rare on any coastline. The website also has a section on the natural history of Port Gaverne and links to other Cornish Natural history sites. -

Forrabury & Minster Voters 1929
Northern Parliamentary Constituency Parish of Forrabury & Minster Register of Electors 1929 Transcribed by Mike Gabriel Abbreviations Men Women R = Residence qualification Rw = Residence qualification B = Business premises qualification BW = Business premises qualification O = Occupation qualification Ow = Occupation qualification D = Qualification through wife's occupation Dw = Qualification through husband's occupation NM = Naval or Military voter No. Forename(s) Surname Residence Franchise Qualifying Property Notes 1 Harry ALFORD Fore Street Parliamentary R; Local Govt. O 2 Kate ALFORD Fore Street Parliamentary Rw; Local Govt. Dw 3 James Henry ALLEN Minster Farm Parliamentary R; Local Govt. O J 4 Susan ALLEN Minster Farm Parliamentary Rw; Local Govt. Dw 5 John Miller ALLEN High Street Parliamentary R; Local Govt. O 6 Violet Mabel ALLEN High Street Parliamentary Rw; Local Govt. Dw 7 Jane ARNOLD Paradise Row Parliamentary Rw; Local Govt. Ow 8 Frederick James ARNOLD 1, Railway Cottages Parliamentary R; Local Govt. ‐ 9 Caroline Emily Gilbee BIASS Glenfinart Parliamentary O; Local Govt. O 10 Russel BAKER Lower Worthyvale Parliamentary R; Local Govt. O J 11 Annie BAKER Lower Worthyvale Parliamentary Rw; Local Govt. Dw 12 Mary Chapman BAKER Lower Worthyvale Parliamentary Rw; Local Govt. Ow 13 Carrie Leonora BAKER Vine Cottage Parliamentary Rw; Local Govt. Ow 14 James BARTLETT Lower Worthyvale Parliamentary R; Local Govt. O 15 Eva BARTLETT Lower Worthyvale Parliamentary Rw; Local Govt. Dw 16 James BARTLETT, Junr. Worthyvale Cottage Parliamentary R; Local Govt. ‐ to vote at Tintagel 17 William BARTLETT Lower Worthyvale Parliamentary R; Local Govt. ‐ 18 Joseph BATH 3, Penally Terrace Parliamentary R; Local Govt. O 19 Elizabeth BATH 3, Penally Terrace Parliamentary Rw; Local Govt. -

Launceston to Bodmin Parkway 10 Via Camelford | Delabole | Port Isaac | Polzeath | Wadebridge | Bodmin
Launceston to Bodmin Parkway 10 via Camelford | Delabole | Port Isaac | Polzeath | Wadebridge | Bodmin Mondays to Saturdays except bank holidays 10S Launceston Westgate St 0710 0740 0925 1125 1325 1325 1515 1535 1740 Launceston College 1525 Tregadillett Primary School 0718 0748 0933 1133 1333 1333 1532 1548 1753 Trethorne Leisure Farm opp 0720 0750 0935 1135 1335 1335 1534 1550 1755 Pipers Pool opp Bus Shelter 0724 0754 0939 1139 1339 1339 1538 1554 1758 Badgall 1545 Tregeare 1547 24 Hallworthy Old Post Office 0731 0801 0946 1146 1346 1346 1600 1601 1601 1805 Trelash 1607 Warbstow Cross 1610 Canworthy Water Chapel 1615 Davidstow opp Church Hall 0735 0805 0950 1150 1350 1350 1605 1605 1808 Arthurian Centre 0740 Delabole Post Office 0745 0745 Weatdowns The Skerries 0750 0750 St Teath Post Office 0756 0756 Helstone Bus Shelter 0800 0800 Camelford Church 0811 0956 1156 1356 1356 1611 1611 1814 Camelford Clease Road 0703 0703 0813 0958 1158 1358 1358 1613 1613 1816 Sir James Smith School 0705 0705 0810 0810 0815 1000 1200 1400 1400 1435 1615 1615 1817 Delabole Post Office 0715 0715 0825 0825 1010 1210 1410 1410 1445 1625 1625 1825 Delabole West Downs Road 0717 0717 0827 0827 1012 1212 1412 1412 1447 1627 1627 1827 Pendoggett Cornish Arms 0726 0726 0836 0836 1021 1221 1421 1421 1456 1636 1636 1836 Port Isaac The Pea Pod 0735 0735 0845 0845 1030 1230 1430 1505 1645 1645 1845 St Endellion Church 0743 0743 0853 0853 1038 1238 1425 1438 1513 1653 1653 1853 Polzeath opp Beach 0755 0755 0755 0905 0905 1050 1250 1437 1450 1705 1705 1905 Rock opp Clock -

East Area V2
to Hartland Welcome Cross Morwenstow Crimp 217 Kilkhampton Coombe Thurdon (school days only) 218 Stibb 218 Poughill 218 217 Bush Grimscott Bude Sea Pool Bude Red Post Stratton Upton Holsworthy Marhamchurch Wednesday only 217 Pyworthy Widemouth Bay Bridgerule Titson Coppathorne Friday only Treskinnick Cross Whitstone Week St Mary North Tamerton Crackington Haven Wainhouse Crackington Corner Monday & Thursday only Broad Boyton Tresparrett Langdon Bennacott Posts Canworthy Water Warbstow Boscastle Langdon North Trevalga Petherwin Trethevy St Nectan's Glen Tintagel Castle Bossiney Yeolmbridge Hallworthy Tintagel Egloskerry Davidstow St Stephen Camelford Trewarmett Station Launceston Castle Pipers Pool East Area Guide Mid Area Guide Launceston Delabole Valley Polyphant Truckle Camelford Altarnun South Helstone Petherwin St Teath Port Isaac Fivelanes Pendoggett Polzeath St Endellion Bodmin Moor Congdon’s Shop Treburley Trelill 236 Trebetherick St Minver Bolventor Pityme St Tudy St Kew Bray Shop Stoke Climsland Rock Highway Bathpool 236 Tavistock Bodmin Moor Rilla Mill 12 12A Linkinhorne Downgate St Mabyn Upton Cross St Ann's Gunnislake to Padstow Kelly Bray Chapel Whitecross Pensilva Wadebridge Callington Harrowbarrow Ashton Calstock River Tamar Bodmin Tremar 12A St Dominick St Neot St Cleer 12 St Ive Ruthernbridge Liskeard Merrymeet St Mellion Rosenannon Winnard’s Doublebois Perch River Tamar Lanivet Bodmin East Taphouse Parkway Menheniot Landrake to Truro St Keyne Tideford Saltash Victoria to Penhale Derriford Hospital Trerulefoot Roche Lostwithiel -

MINUTES of the FULL COUNCIL MEETING HELD in PORT ISAAC SCHOOL MONDAY, 9Th OCTOBER 2017 @ 7Pm Present: Cllr
MINUTES OF THE FULL COUNCIL MEETING HELD IN PORT ISAAC SCHOOL th MONDAY, 9 OCTOBER 2017 @ 7pm Present: Cllr. Raynor (Chairman) Cllr. Dawe Cllr. Penny Cllr. Webster Cllr. Williams Mrs Thompson (Clerk) County Cllr. Mould Minute AGENDA ITEMS Action Chairman’s Welcome and Public Forum – the Chairman welcomed those present and advised of exits, local hazards, location of WCs, etc. The Chairman invited the members of the public to speak. Dr Sainsbury said he did not think the application to build a house at Archer Farm (Minute 211h/2017 refers) met with the requirements of the Cornwall Local Plan. He pointed out the site was in the AONB. He also felt, with others, that the access was not suitable as the lane was narrow and unmade. Mrs Sainsbury supported her husband’s comments. Mrs Dora Wainwright commented she thought there shouldn’t be further development down into the valley. Mr Tony Wainwright added his agreement and said that in his opinion further development into the valley was inappropriate and would set a precedent. Mrs Vernon and her daughter were present to support their application to build a dwelling on land NW of Trecreege Farm (Minute 211g/2017 refers). This was for an agricultural dwelling. Mrs Penny Kirkman supported the proposition in principle but thought the site was too high and would cause harm to the AONB. Mrs Jenny Railton added access was poor, the track over her land was unmade and would not sustain heavy use. These two members of the public were against this application as they believed it to be too far from the main farm and the site is in an AONB. -

'Henwyn Kath' / Cat Names
‘Henwyn Kath’ / Cat Names Some fun ideas: Name in Cornish Name in English Queen / Tom Brewer Bruiser Queen / Tom Brithel Mackerel Queen / Tom Dreysen Bramble Queen Gourgath Tom-cat Tom Gwandrek Wandering Tom Gwandrik Wandering Queen Kath Cat Queen Kathik Kitten, pussy Queen Komolen Single cloud Queen Kynsa First Queen / Tom Lewik Lion cub Queen / Tom Lewpard Leopard Queen / Tom Margh Mark Tom Morgath Sea cat Queen / Tom Nessa Second Queen / Tom Steren Star Queen Tikki Duw Butterfly Queen / Tom Tressa Third Queen / Tom Attributes and characteristics: Name in Cornish Name in English Queen / Tom Bras Big Queen / Tom Bros Gross Queen / Tom Bian Small Queen / Tom Blewek Fluffy Queen / Tom Brithek Stripy Tom Brithik Stripy Queen Bryghek Spotty Tom Bryghik Spotty Queen Byghan Little Queen / Tom Duik Little black Queen / Tom Erghek Snowy Tom Erghik Snowy Queen Gwynnik Little white Queen / Tom 1. ‘Henwyn Kath’ / Cat Names Koskek Sleepy Tom Koskik Sleepy Queen Lagasek Big eyed Queen / Tom Lodrow Socks Queen / Tom Logoser Mouser Queen / Tom Losik Greyish Queen / Tom Melek Honey colour Tom Melik Honey colour Queen Mog Smoke Queen / Tom Cornish equivalents of English first names: Name in Cornish Name in English Queen / Tom Arthur Arthur Tom Davydh David Tom Eler Hilary Queen / Tom Eppow Elizabeth Queen Eva Eve Queen Gawen Gavin Tom Gwenna Gwen Queen Jenna Jane Queen Jori George Tom Jowan John Tom Katel Catherine Queen Lowena Joy Queen Maria Mary Queen Myghal Michael Tom Neghtan Nathan Tom Peder Peter Tom Pedrek Petroc Tom Rosen Rose Queen Rosennik Rosie Queen Stefan Stephen Tom Tommik Tommy Tom Budhek Victor Tom Budhik Victoria Queen Wella William Tom Yosep Joseph Tom 2. -

Forrabury & Minster Voters 1927
Register of Electors 1927 Northern Parliamentary Constituency, County of Cornwall Polling District of Boscastle (B) Parish of Forrabury and Minster Transcribed by Mike Gabriel Abbreviations R = Residence qualification BP = Business premises qualification O = Occupation qualification HO = Qualification through husband's occupation NM = Naval, Military or Air Force Voter SJ = person qualified for and liable to serve as Special Juror J = person qualified for and liable to serve as Juror No. Forename(s) Surname Residence Franchise Qualifying Property Notes 1 Harry ALFORD High Street Parliamentary R ; Local Govt. O 2 Kate ALFORD High Street Parliamentary HO ; Local Govt. HO 3 James Henry ALLEN Small Holding Parliamentary R ; Local Govt. O J 4 Susan ALLEN Small Holding Parliamentary HO ; Local Govt. HO 5 John Miller ALLEN High Street Parliamentary R ; Local Govt. O 6 Jane ARNOLD Paradise Row Parliamentary O ; Local Govt. O 7 George ARSCOTT Wellington Hotel Parliamentary R ; Local Govt. ‐ 8 Caroline Emily Gilbee BAISS Glenfinart Parliamentary O ; Local Govt. O 9 Russel BAKER Lower Worthyvale Parliamentary R ; Local Govt. O J 10 Annie BAKER Lower Worthyvale Parliamentary HO ; Local Govt. HO 11 Mary Chapman BAKER Lower Worthyvale Parliamentary O ; Local Govt. O 12 James BARTLETT Lower Worthyvale Parliamentary R ; Local Govt. O 13 Eva BARTLETT Lower Worthyvale Parliamentary HO ; Local Govt. HO 14 James BARTLETT, Junr. Worthyvale Cottage Parliamentary R ; Local Govt. ‐ 15 Margaret BARTON Welltown Parliamentary O ; Local Govt. O 16 Joseph BATH Penally Terrace Parliamentary R ; Local Govt. O 17 Elizabeth BATH Penally Terrace Parliamentary HO ; Local Govt. HO 18 James BATH Sunnyside Parliamentary R ; Local Govt. -

Wadebridge | Rock | Polzeath | Port Isaac | Camelford
Wadebridge | Rock | Polzeath | Port Isaac | Camelford Camelford | Port Isaac | Polzeath | Rock | Wadebridge 96 Mondays to Saturdays except public holidays Mondays to Saturdays except public holidays Wadebridge bus station dep 0835 1120 1235 1506 1735 Delabole Smugglers 0721 0931 1346 1616 Wadebridge The Platt 0837 1122 1237 1508 1737 Delabole Westdowns Road 0725 0935 1350 1620 Wadebridge school 0840 1125 1240 1511 1740 Pendoggett Cornish Arms 0732 0942 1357 1627 St Minver Fourways Inn 0850 1135 1250 1521 1750 Port Isaac church hall rooms 0738 0948 1403 1633 Tredrizzick Pityme Inn 0854 1139 1254 1525 1754 St Endellion church 0746 0956 1411 1641 Rock garage 0857 1142 1257 1528 1757 Polzeath beach 0757 1007 1157 1422 1652 Trebetherick The Mowhay 0902 1147 1302 1533 1802 Trebetherick opp The Mowhay 0802 1012 1202 1427 1657 Polzeath beach 0907 1152 1307 1538 1807 Rock garage 0807 1017 1207 1432 1702 St Endellion church 0918 1318 1549 1818 Tredrizzick post box 0810 1020 1210 1435 1705 Port Isaac church hall rooms 0925 1325 1556 1825 St Minver Fourways Inn 0814 1024 1214 1439 1709 Pendoggett Cornish Arms 0932 1332 1603 1832 Wadebridge opp school 0824 1034 1224 1449 1719 Delabole Post Office 0939 1339 1610 1839 Wadebridge The Platt 0827 1037 1227 1452 1722 Delabole Smugglers 0943 1343 1614 1843 Wadebridge bus station arr 0829 1039 1229 1454 1724 this journey runs from 8 July until 31 August connect with route 95 from Truro or Newquay at Wadebridge bus station this bus continues to Camelford & Bodmin as route 55 this bus starts in Camelford & -

Forrabury and Minster Housing Needs Assessment (HNA)
Forrabury and Minster Housing Needs Assessment (HNA) For Forrabury and Minster Neighbourhood Plan 2021- 2040 December 2020 AECOM Forrabury and Minster Neighbourhood Plan Housing Needs Assessment 2 Quality information Prepared by Checked by Approved by Elena Butterworth Paul Avery Paul Avery Graduate Planner Senior Consultant Senior Consultant Revision History Revision Revision date Details Authorized Name Position 1 October 2020 First draft EB Elena Graduate Butterworth Planner 2 October 2020 Technical review PA Paul Avery Senior Consultant 3 October 2020 Second draft EB Elena Graduate Butterworth Planner 4 October 2020 Group review SD Sally Dickinson Neighbourhood Planning Group 5 November 2020 Third draft PA Paul Avery Senior Consultant 6 December 2020 Locality review AO Annabel Neighbourhood Osborne Planning Officer 7 December 2020 Final report PA Paul Avery Senior Consultant AECOM Forrabury and Minster Neighbourhood Plan Housing Needs Assessment 3 Prepared for: Forrabury and Minster Parish Council Prepared by: Elena Butterworth AECOM Aldgate Tower 2 Leman Street London E1 8FA aecom.com © 2020 AECOM. All Rights Reserved. This document has been prepared by AECOM Limited (“AECOM”) in accordance with its contract with Locality (the “Client”) and in accordance with generally accepted consultancy principles, the budget for fees and the terms of reference agreed between AECOM and the Client. Any information provided by third parties and referred to herein has not been checked or verified by AECOM, unless otherwise expressly stated in the document. AECOM shall have no liability to any third party that makes use of or relies upon this document. Disclaimer This document is intended to aid the preparation of the Neighbourhood Plan, and can be used to guide decision making and as evidence to support Plan policies, if the Qualifying Body (i.e. -

Election of Town and Parish Councillors Notice Is Hereby Given That 1
Notice of Election Election of Town and Parish Councillors Notice is hereby given that 1. Elections are to be held of Town and Parish Councillors for each of the under-mentioned Town and Parish Councils. If the elections are contested the poll will take place on Thursday 2 May, 2013. 2. I have appointed Geoff Waxman, Sharon Holland and John Simmons whose offices are Room 33, Cornwall Council, Luxstowe House, Liskeard, PL14 3DZ to be my Deputies and are specifically responsible for the following Town and Parishes: Town / Parish Seats Town / Parish Seats Town / Parish Seats Altarnun 6 Maker with Rame 11 St Eval 7 Antony 6 Marhamchurch 10 St Ewe 10 Blisland 10 Mawgan-in-Pydar (St. Mawgan Ward) 6 St Gennys 10 Bodmin (St Leonard Ward) 5 Mawgan-in-Pydar (Trenance Ward) 6 St Germans (Bethany Ward) 2 Bodmin (St Mary's Ward) 6 Menheniot 11 St Germans (Polbathic Ward) 2 Bodmin (St Petroc Ward) 5 Mevagissey 14 St Germans (St Germans Ward) 4 Botus Fleming 8 Michaelstow 5 St Germans (Tideford Ward) 3 Boyton 8 Millbrook 13 St Goran 10 Bude-Stratton (Bude Ward) 9 Morval 10 St Issey 10 Bude-Stratton (Flexbury and Poughill Ward) 6 Morwenstow 10 St Ive (Pensilva Ward) 10 Bude-Stratton (Stratton Ward) 3 Newquay (Newquay Central Ward) 3 St Ive (St Ive Ward) 3 Callington (Callington Ward) 10 Newquay (Newquay Pentire Ward) 4 St John 6 Callington (Kelly Bray Ward) 2 Newquay (Newquay Treloggan Ward) 4 St Juliot 5 Calstock (Calstock Ward) 3 Newquay (Newquay Tretherras Ward) 3 St Kew (Pendoggett Ward) 1 Calstock (Chilsworthy Ward) 2 Newquay (Newquay Treviglas