The Molluscicidal Effects of Hyptis Suaveolens on Different Stages of Bulinus Globosus in the Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ORNAMENTAL GARDEN PLANTS of the GUIANAS: an Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana
f ORNAMENTAL GARDEN PLANTS OF THE GUIANAS: An Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana Vf•-L - - •• -> 3H. .. h’ - — - ' - - V ' " " - 1« 7-. .. -JZ = IS^ X : TST~ .isf *“**2-rt * * , ' . / * 1 f f r m f l r l. Robert A. DeFilipps D e p a r t m e n t o f B o t a n y Smithsonian Institution, Washington, D.C. \ 1 9 9 2 ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Table of Contents I. Map of the Guianas II. Introduction 1 III. Basic Bibliography 14 IV. Acknowledgements 17 V. Maps of Guyana, Surinam and French Guiana VI. Ornamental Garden Plants of the Guianas Gymnosperms 19 Dicotyledons 24 Monocotyledons 205 VII. Title Page, Maps and Plates Credits 319 VIII. Illustration Credits 321 IX. Common Names Index 345 X. Scientific Names Index 353 XI. Endpiece ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Introduction I. Historical Setting of the Guianan Plant Heritage The Guianas are embedded high in the green shoulder of northern South America, an area once known as the "Wild Coast". They are the only non-Latin American countries in South America, and are situated just north of the Equator in a configuration with the Amazon River of Brazil to the south and the Orinoco River of Venezuela to the west. The three Guianas comprise, from west to east, the countries of Guyana (area: 83,000 square miles; capital: Georgetown), Surinam (area: 63, 037 square miles; capital: Paramaribo) and French Guiana (area: 34, 740 square miles; capital: Cayenne). Perhaps the earliest physical contact between Europeans and the present-day Guianas occurred in 1500 when the Spanish navigator Vincente Yanez Pinzon, after discovering the Amazon River, sailed northwest and entered the Oyapock River, which is now the eastern boundary of French Guiana. -

Dock and Crop Images
orders: [email protected] (un)subscribe: [email protected] Current Availability for September 25, 2021 Dock and Crop images Click any thumbnail below for the slideshow of what we shipped this past week: CYCS ARE RED HOT GIANT GLOSSY LEAVES BLUE MOONSCAPE SUCCULENT BLUE LEAVES SUCCULENT ORANGE LEAVES SPECKLED LEAVES CYCS ARE RED HOT RED SUNSETSCAPE Jeff's updates - 9/16 dedicated this week's favorites Chimi's favorite climbing structure 4FL = 4" pot, 15 per flat 10H = 10" hanging basket n = new to the list ys = young stock 6FL = 6" pot, 6 per flat 10DP = 10" Deco Pot, round b&b = bud and bloom few = grab 'em! QT= quart pot, 12 or 16 per flat nb = no bloom * = nice ** = very nice Quarts - 12 per flat, Four Inch - 15 per flat, no split flats, all prices NET code size name comments comments 19406 4FL Acalypha wilkesiana 'Bronze Pink' ** Copper Plant-colorful lvs 12210 QT Acorus gramineus 'Ogon' ** lvs striped creamy yellow 19069 4FL Actiniopteris australis ** Eyelash Fern, Ray Fern 17748 4FL Adiantum hispidulum ** Rosy Maidenhair 17002 4FL Adiantum raddianum 'Microphyllum' ** extremely tiny leaflets 21496 4FL Adromischus filicaulis (cristatus?) ** Crinkle Leaf 16514 4FL Aeonium 'Kiwi' ** tricolor leaves 13632 QT Ajuga 'Catlin's Giant' ** huge lvs, purple fls 13279 QT Ajuga pyramidalis 'Metallica Crispa' ** crinkled leaf 17560 4FL Aloe vera * Healing Aloe, a must-have 13232 QT Anthericum sanderii 'Variegated' *b&b grassy perennial 13227 QT Asparagus densiflorus 'Meyer's' ** Foxtail Fern 19161 4FL Asplenium 'Austral Gem' -

Effect of Plant Growth Enhancing Substances on Plant Architecture of Euphorbia Milii Var
Journal of Pharmacognosy and Phytochemistry 2017; 6(6): 742-748 E-ISSN: 2278-4136 P-ISSN: 2349-8234 Effect of plant growth enhancing substances on plant JPP 2017; 6(6): 742-748 Received: 22-09-2017 architecture of Euphorbia milii var. ‘Pink Bold Beauty’ Accepted: 24-10-2017 Kapadiya DB Kapadiya DB, Alka Singh, Bhandari AJ and Ahlawat TR Research Scholar, Department of Floriculture and LA, ACHF, NAU, Navsari, Gujarat, India Abstract The investigation aimed to study the effect of plant growth enhancing substances on plant canopy, Alka Singh vegetative growth and flowering as well as on overall appearance of Euphorbia milii plants grown in pot Research Scholar, Department of during 2015 – 2017. Application of silicon, spermine and salicylic acid at different concentrations Floriculture and LA, ACHF, significantly influenced the growth, flowering, pigments as well as overall appearance of Euphorbia milii NAU, Navsari, Gujarat, India plants during both the seasons as compared to untreated plants (control). Plants treated with 300 mg/l silicon and 3.0 mg/l salicylic acid showed maximum plant height, plant spread, number of branches with Bhandari AJ Research Scholar, Department of thicker stems and maximum number of leaves with increased leaf area during experiment. Early flower Floriculture and LA, ACHF, bud initiation (19.35 & 20.46 days) and flower opening (7.60 & 7.85 days) of Euphorbia flowers was NAU, Navsari, Gujarat, India observed with application of spermine at 30 mg/l. Maximum number of inflorescence per plant with increased inflorescence diameter, flowers per inflorescence with improved flower size were obtained Ahlawat TR from plants treated with 3.0 mg/l salicylic acid as recorded at 30, 60 and 90 DAS. -

On Euphorbia Milii (Euphorbiaceae) and Its Varieties Jean-Philippe Castillon & Jean-Bernard Castillon
On Euphorbia milii (Euphorbiaceae) and its varieties Jean-Philippe Castillon & Jean-Bernard Castillon Fig. 1: Drawing of Euphorbia milii taken from the original de- Fig. 2: Drawing of Euphorbia bojeri, being the type of the species scription (Des Moulins, 1826) that is a synonym of Euphorbia milii (taken from W. J. Hooker, 1836, Curtis’s Botanical Magazine, Vol. 63: t. 3527, reproduced with per- mission © the Board of Trustees of the Royal Botanic Gardens, Kew) uphorbia milii Des Moulins is at this time the ated for thorny spurges with large red cyathophylls best known of the Madagascan spurges – the one from Madagascar (E. splendens Bojer ex Hook., E. bojeri that is called “crown of thorns” (“la couronne Hook., E. hislopii N.E.Br., E. breonii Nois.), that nowa- Ed’épines” in French), grown in a large number of botani- days are considered as synonyms or varieties of E. milii. cal gardens and by succulent amateurs – and one of the Even after the rediscovery of Des Moulins’ publication most poorly defined from a taxonomical point of view. and the rehabilitation of the name E. milii the true plant It was described by Charles Des Moulins (1826) from to which this name was applied remained unknown. As living plants brought back to Paris by Baron Milius in a consequence many authors preferred to create varieties 1821, but without type material or type locality. The of E. milii rather than establish new species for plants publication of Des Moulins was subsequently completely resembling the enigmatic E. milii, even though they ignored or forgotten. -

CROWN of THORNS Euphorbia Milii Characteristics Culture Noteworthy
CROWN OF THORNS Euphorbia milii Characteristics Type: Houseplant Water: Dry to medium Zone: 9 to 11 Maintenance: Medium Height: 3.00 to 6.00 feet Flower: Showy Spread: 1.50 to 3.00 feet Leaf: Evergreen Bloom Time: Seasonal bloomer Other: Thorns Bloom: Green subtended by red or yellow Tolerate: Rabbit, Deer, Drought, Dry Soil, bracts Air Pollution Sun: Full sun, part shade Culture Winter hardy to USDA Zones 9-11 where plants are best grown in dry to medium moisture, well-drained soils in full sun. Plants react poorly to temperatures that dip below 35 degrees F. in winter. Can be grown in pots outside and brought indoors in fall. Appreciates some midday shade in hot summer climates. Plants are tolerant of poor soils, including rocky-sandy ones. Plants are also tolerant of dry soils, but regular applications of moderate moisture may result in better bloom with less leaf drop. Wet soils, particularly in winter, can be fatal. Best located in areas with good air circulation. Indoor plants need bright light and are best grown with a gritty soil-based potting mix. Propagate from tip cuttings. Wear gloves when working with this plant. Sticky white latex sap is poisonous (avoid contact with skin, mouth or eyes). Noteworthy Characteristics Euphorbia milii, commonly called crown of thorns, is a woody, succulent shrub that features (a) fleshy, bright green leaves, (b) inconspicuous flowers in clusters subtended by very showy petal-like red or yellow bracts and (c) thick sharp black thorns (to 1/2" long) which cover its water-storing branches and stems. -

The Optimal Dose of Euphorbia Milii Extracts in Nkp46 Expression Against Mice Infected with Mycobacterium Tuberculosis
Apr.-Jun. 2014, Volume 11, No. 2 (Serial No. 94) pp. 68-73 D Journal of US-China Medical Science, ISSN 1548-6648, USA D AV I D PUBLISHING The Optimal Dose of Euphorbia milii Extracts in NKp46 Expression against Mice Infected with Mycobacterium tuberculosis Ni Made Linawati1, Ida Ayu Alit Widhiartini2, I Nyoman Wande3, Ida Bagus Nyoman Putra Dwija4, I Gusti Sri Wiryawan1, I Gusti Kamasan Arijana1, Wayan Sugiritama1, I Gusti Ayu Ratnayanti1, Ida Ayu Ika Wahyuniari1 and I Gusti Ngurah Mayun1 1. Department of Histology, Faculty of Medicine, University of Udayana, Denpasar 80232, Bali, Indonesia 2. Department of Pharmacy, Faculty of Medicine, University of Udayana, Denpasar 80232, Bali, Indonesia 3. Department of Clinical Patology, Faculty of Medicine, University of Udayana, Denpasar 80232, Bali, Indonesia 4. Department of Microbiology, Faculty of Medicine, University of Udayana, Denpasar 80232, Bali, Indonesia Abstract: Objective: To compare the effect of EEM (Euphorbia milii) extract in various dose for NKp46 expression in mice infected with MTB (Mycobacterium tuberculosis). Methods: An experimental study with 24 mice Balbc were divided into 8 groups of treatment which is observed at weeks I and II. All groups were infected with Mycobacterium tuberculosis strain H37Rv then each successive group was given sterile distilled water (P0.1 and P0.2), extract of EEM 5 mg/20 grbw (gram body weight) (P1.1 and P1.2), 10 mg/20 grbw (P2.1 and P2.2), and 15 mg/20 grbw (P3.1 and P3.2). Then, termination in each group at weeks I (P0.1, P1.1, P2.1 and P3.1) and II (P0.2, P1.2, P2.2 and P3.2). -

PHYTOCHEMICAL SCREENING and ANTIMICROBIAL ACTIVITY of FLOWER EXTRACT of Euphorbia Milii
BMR Phytomedicine www.advancejournals.org Open Access Scientific Publisher Research Article PHYTOCHEMICAL SCREENING AND ANTIMICROBIAL ACTIVITY OF FLOWER EXTRACT OF Euphorbia Milii Megha Vyas1, Binita Desai1 1Department of microbiology, Shree Ramakrishna Institute of computer education And Applied Sciences, Atwalines, Surat-395 001, India Correspondence should be addressed to Megha Vyas Received February 27, 2018; Accepted March 31, 2018; Published April 07, 2018; Copyright: © 2018 Megha Vyas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Cite This Article: Vyas, M., Desai, B.(2018). Phytochemical screening and antimicrobial activity of flower extract of Euphorbia milii. BMR Phytomedicine, 4(1). 1-6 ABSTRACT An ornamental plant Euphorbia milii (Euphorbiaceae) is plays a role in folk medicine. The Chinese use it as a cure for cancer, and some Brazilians believe that it can cure warts. Phytochemical studies showed the presence of cardiac glycosides, steroids/phytosterols, anthocyanin, terpenoids, flavonoids and tannins.The Antimicrobial activity studies of hexane, ethyl acetate,chloform,petroleum ether,and water extracts of flowers of Euphorbia milii were performed on gram positive organisms(Bacillus subtilis, Bacillus megaterium,Staphylococcus aureus, Entrococci ) and gram negative organisms (Escherichia coli and Proteus vulgaris, Pusodomonas aeruginosa) by using cup plate method. The hexane extracts in the concentration of 5μg/ml, have shown considerable inhibition zone on as compared to other extracts.Bacillus subtlis and Bacillus megaterium have not show inhibition zone from solvent extract which was compared with the inhibition zone produced by standard amikacin sulphate (1μg/ml.). -

Crown-Of-Thorns, Euphorbia Milii Ch. Des Moulins, Is a Tender Semi-Evergreen Which Is Widely Grown in South Florida
Plant Pathology Circular No. 134 Fla. Dept. Agr. & Consumer Serv., September 1973 Division of Plant Industry Bacterial Leaf Spot of Crown-of-Thorns J. W. Miller Crown-of-thorns, Euphorbia milii Ch. des Moulins, is a tender semi-evergreen which is widely grown in South Florida. It flowers freely throughout the year but more abundantly during the winter. The leaves are sparse on this thorny plant, and the flowers are arranged in long-stalked clusters sur— rounded by red bracts. The plant is used in border plantings, in rock gar— dens and planter boxes, and as specimen plants (2). Bacterial leaf spot, caused by Xanthomonas poinsettiaecola Patel, was first found on crown-of-thorns in Florida in 1966 (1) and has become a common and serious disease. The disease is characterized by circular gray to brown leaf spots surrounded by distinct yellow halos. The infected leaves often turn yellow and fall off the plants (3). These leaf spots and sparse foliage make the plants unsightly and unsalable. Poinsettia (Euphorbia pulcherrima Willd.) and wild poinsettia (Euphorbia heterophylla L.) are also hosts and the disease may be transmitted readily from one to the other. The bacteria are spread by splashing rain or overhead irrigation and also by diseased cuttings. CONTROL. Disease control is best attained by using disease-free cuttings, removing diseased leaves or plants when they are found, and keeping the leaves as dry as possible. Agri-strep at 200 ppm streptomycin sulfate or Fig. 1. Xanthomonas poinsettiaecola on crown-of-thorns displaying brown leaf spots surrounded by irregular yellow halo. -

12Th Annual UF/IFAS Extension Sarasota County Master Gardener Plant Sale
12th Annual UF/IFAS Extension Sarasota County Master Gardener Plant Sale Common Name Botanical Name Staging Area African Blue Basil Ocimum kilimandscharicum x basilicum 'Dark Opal' Butterfly African Milk Bush Synadenium grantii Tropical African or Butterfly Iris Dietes iridioides (formerly vegeta) Ornamental Agave, Century Plant Agave americana 'Marginata' Tropical Agave, Variegated Agave angustifolia Tropical Aloe Aloe vera Tropical Amaryllis, Red Amaryllis sp Tropical Angel Trumpet, Peach Brugmansia X candida sp. Ornamental Anthurium Anthurium sp. Tropical Australian Violet Viola hederacea Groundcover Baby Sunrose Aptenia cordifolia Tropical Bald Cypress Taxodium distichum Tree/Shrub Bamboo, clumping Bambusa sp. Ornamental Bamburanta Prayer Plant Ctenanthe compressa Groundcover Banana, 'Nam Wah' Musa 'Nam Wah' Edible Basket Plant Callisia fragrans Groundcover Bay Rum Pimenta racemosa Tree/Shrub Beach Sunflower Helianthus debilis Ornamental Beautyberry Callicarpa americana Tree/Shrub Begonia, Angel Wing Begonia, Angel Wing Tropical Begonia, Sweet Begonia odorata 'Alba' Tropical Begonia, White Begonia popenoei Tropical Bird of Paradise Strelitzia reginae Tropical Bird of Paradise, White Strelitzia nicolai Tropical Blackberry Rubus sp. Edible Blackberry Jam Fruit Randia formosa Edible Blanket Flower Gaillardia pulchella Butterfly Bleeding Heart Vine Clerodendrum thomsoniae Tropical Blue Daze Evolvulus glomeratus Groundcover Blue Mist Flower Conoclinium coelestinum Ornamental Blue Pea Vine Clitoria ternatea Vine Blue Sage Eranthemeum pulchellum -
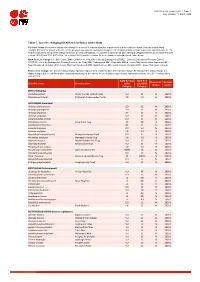
Table 7: Species Changing IUCN Red List Status (2018-2020)
IUCN Red List version 2020-1: Table 7 Last Updated: 19 March 2020 Table 7: Species changing IUCN Red List Status (2018-2020) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2019 (IUCN Red List version 2019-3) and 2020 (IUCN Red List version 2020-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2019) List (2020) change version Category -

Lepidoptera: Gracillariidae): an Adventive Herbivore of Chinese Tallowtree (Malpighiales: Euphorbiaceae) J
Host range of Caloptilia triadicae (Lepidoptera: Gracillariidae): an adventive herbivore of Chinese tallowtree (Malpighiales: Euphorbiaceae) J. G. Duncan1, M. S. Steininger1, S. A. Wright1, G. S. Wheeler2,* Chinese tallowtree, Triadica sebifera (L.) Small (Malpighiales: Eu- and the defoliating mothGadirtha fusca Pogue (Lepidoptera: Nolidae), phorbiaceae), native to China, is one of the most aggressive and wide- both being tested in quarantine to determine suitability for biological spread invasive weeds in temperate forests and marshlands of the control (Huang et al. 2011; Wang et al. 2012b; Pogue 2014). The com- southeastern USA (Bruce et al. 1997). Chinese tallowtree (hereafter patibility of these potential agents with one another and other herbi- “tallow”) was estimated to cover nearly 185,000 ha of southern for- vores like C. triadicae is being examined. The goal of this study was to ests (Invasive.org 2015). Since its introduction, the weed has been re- determine if C. triadicae posed a threat to other native or ornamental ported primarily in 10 states including North Carolina, South Carolina, plants of the southeastern USA. Georgia, Florida, Alabama, Mississippi, Louisiana, Arkansas, Texas, and Plants. Tallow plant material was field collected as seeds, seed- California (EddMapS 2015). Tallow is now a prohibited noxious weed lings, or small plants in Alachua County, Florida, and cultured as pot- in Florida, Louisiana, Mississippi, and Texas (USDA/NRCS 2015). As the ted plants and maintained in a secure area at the Florida Department existing range of tallow is expected to increase, the projected timber of Agriculture and Consumer Services, Division of Plant Industry. Ad- loss, survey, and control costs will also increase. -

Euphorbia Milii Des Moul
CPVO/TP-091/1 Date: 01/12/2005 European Union Community Plant Variety Office PROTOCOL FOR DISTINCTNESS, UNIFORMITY AND STABILITY TESTS Euphorbia milii Des Moul. & its hybrids CROWN OF THORNS UPOV Species Code: EUPHO_MIL Adopted on 1st December 2005 1 CPVO/TP-091/1 Date: 01/12/2005 I - SUBJECT OF THE PROTOCOL The protocol describes the technical procedures to be followed in order to meet the requirement of Council Regulation 2100/94 on Community Plant Variety Rights. The technical procedures have been agreed by the Administrative Council and are based on general UPOV Document TG/1/3 and UPOV Guideline TG/91/3 dated 07/11/1984 for the conduct of tests for Distinctness, Uniformity and Stability. This protocol applies to all vegetatively propagated varieties of Euphorbia milii Des Moul. and its hybrids of the family Euphorbiaceae. II - SUBMISSION OF PLANT MATERIAL 1. The Community Plant Variety Office (CPVO) is responsible for informing the applicant of: • the closing date for the receipt of plant material; • the minimum amount and quality of plant material required; • the examination office to which material is to be sent. The applicant is responsible for ensuring compliance with any customs and plant health requirements. 2. Final dates for receipt of documentation and material by the Examination Office The final dates for receipt of requests, technical questionnaires and the final date or submission period for plant material will be decided by the CPVO and each Examination Office chosen. The Examination Office is responsible for immediately acknowledging the receipt of requests for testing, and technical questionnaires. If no or unsatisfactory plant material is submitted the CPVO should be informed as soon as possible.