In T Cell Activity. Effects of Selective PDE7 Inhibitors and Dual PDE4/7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functional T Cells Phosphodiesterase 7A-Deficient Mice Have
Phosphodiesterase 7A-Deficient Mice Have Functional T Cells Guchen Yang, Kim W. McIntyre, Robert M. Townsend, Henry H. Shen, William J. Pitts, John H. Dodd, Steven G. This information is current as Nadler, Murray McKinnon and Andrew J. Watson of October 2, 2021. J Immunol 2003; 171:6414-6420; ; doi: 10.4049/jimmunol.171.12.6414 http://www.jimmunol.org/content/171/12/6414 Downloaded from References This article cites 37 articles, 17 of which you can access for free at: http://www.jimmunol.org/content/171/12/6414.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on October 2, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2003 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Phosphodiesterase 7A-Deficient Mice Have Functional T Cells Guchen Yang,1 Kim W. McIntyre, Robert M. Townsend, Henry H. Shen, William J. Pitts, John H. Dodd, Steven G. Nadler, Murray McKinnon, and Andrew J. -

Inhibiting PDE7A Enhances the Protective Effects of Neural Stem
Research Article: New Research | Cognition and Behavior Inhibiting PDE7A enhances the protective effects of neural stem cells on neurodegeneration and memory deficits in sevoflurane-exposed mice https://doi.org/10.1523/ENEURO.0071-21.2021 Cite as: eNeuro 2021; 10.1523/ENEURO.0071-21.2021 Received: 19 February 2021 Revised: 21 May 2021 Accepted: 25 May 2021 This Early Release article has been peer-reviewed and accepted, but has not been through the composition and copyediting processes. The final version may differ slightly in style or formatting and will contain links to any extended data. Alerts: Sign up at www.eneuro.org/alerts to receive customized email alerts when the fully formatted version of this article is published. Copyright © 2021 Huang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license, which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed. 1 Inhibiting PDE7A enhances the protective effects of neural stem cells on 2 neurodegeneration and memory deficits in sevoflurane-exposed mice 3 Yanfang Huang, Yingle Chen*, Zhenming Kang, Shunyuan Li* 4 Department of Anesthesiology, Quanzhou First Hospital Affiliated to Fujian Medical 5 University, Quanzhou 362000, Fujian, China 6 7 *Corresponding authors 8 Shunyuan Li and Yingle Chen 9 Department of Anesthesiology, Quanzhou First Hospital Affiliated to Fujian Medical 10 University, Quanzhou 362000, Fujian, China 11 Email: [email protected] (Shunyuan Li); [email protected] (Yingle Chen) 12 Tel: 86-18960333666 13 14 15 Running title: Role of PDE7A in neurodegeneration 16 17 1 18 Abstract 19 Sevoflurane is widely used in general anesthesia, especially for children. -

Mechanisms of Action of PDE5 Inhibition in Erectile Dysfunction
International Journal of Impotence Research (2004) 16, S4–S7 & 2004 Nature Publishing Group All rights reserved 0955-9930/04 $30.00 www.nature.com/ijir Original Research Mechanisms of action of PDE5 inhibition in erectile dysfunction JD Corbin1* 1Department of Molecular Physiology and Biophysics, Vanderbilt University School of Medicine, Nashville, Tennesse, USA A spinal reflex and the L-arginine–nitric oxide–guanylyl cyclase–cyclic guanosine monophosphate (cGMP) pathway mediate smooth muscle relaxation that results in penile erection. Nerves and endothelial cells directly release nitric oxide in the penis, where it stimulates guanylyl cyclase to produce cGMP and lowers intracellular calcium levels. This triggers relaxation of arterial and trabecular smooth muscle, leading to arterial dilatation, venous constriction, and erection. Phosphodiesterase 5 (PDE5) is the predominant phosphodiesterase in the corpus cavernosum. The catalytic site of PDE5 normally degrades cGMP, and PDE5 inhibitors such as sildenafil potentiate endogenous increases in cGMP by inhibiting its breakdown at the catalytic site. Phosphorylation of PDE5 increases its enzymatic activity as well as the affinity of its allosteric (noncatalytic/GAF domains) sites for cGMP. Binding of cGMP to the allosteric site further stimulates enzymatic activity. Thus phosphorylation of PDE5 and binding of cGMP to the noncatalytic sites mediate negative feedback regulation of the cGMP pathway. International Journal of Impotence Research (2004) 16, S4–S7. doi:10.1038/sj.ijir.3901205 Keywords: phosphodiesterase inhibitors; vasodilator agents; cyclic GMP; impotence; penile erection Introduction the tone of penile vasculature and the smooth muscle of the corpus cavernosum. In primates, including humans, the L-arginine– In recent years, a deeper understanding of the nitric oxide–guanylyl cyclase–cyclic guanosine regulation of penile smooth muscle has led to monophosphate (cGMP) pathway is the key me- greater insight into the physiology of normal erectile chanism of penile erection1–4 (Figure 1). -
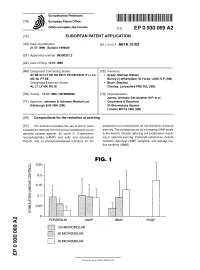
Compositions for the Reduction of Scarring
^ ^ ^ ^ I ^ ^ ^ ^ II ^ II ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ I ^ � European Patent Office Office europeen des brevets EP 0 930 069 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) |nt C|.6: A61 K 31/52 21.07.1999 Bulletin 1999/29 (21) Application number: 99300201.3 (22) Date of filing: 12.01.1999 (84) Designated Contracting States: (72) Inventors: AT BE CH CY DE DK ES Fl FR GB GR IE IT LI LU • Grady, Michael William MC NL PT SE Burley-in-Wharfedale, W.Yorks. LS29 7LP (GB) Designated Extension States: • Bloor, Stephen AL LT LV MK RO SI Chorley, Lancashire PR6 7QL (GB) (30) Priority: 13.01.1998 GB 9800640 (74) Representative: James, Anthony Christopher W.P. et al (71) Applicant: Johnson & Johnson Medical Ltd. Carpmaels & Ransford Edinburgh EH2 4NH (GB) 43 Bloomsbury Square London WC1A2RA (GB) (54) Compositions for the reduction of scarring (57) The invention provides the use of one or more preparation of a composition for the reduction of wound substances selected from the group consisting of (a) an scarring. The substances act by increasing cAMP levels adenylyl cyclase agonist, (b) cyclic 3', 5'-adenosine in the wound, thereby reducing cell proliferation result- monophosphate (cAMP) and salts and derivatives ing in reduced scarring. Preferred substances include thereof, and (c) phosphodiesterase inhibitors for the forskolin, dybutyryl cAMP, lisofylline, and isobutyl me- thyl xanthine (IBMX). FIG. 1 0.25-1 o I- z: o 0.2 H o LU > o 0.15H CD < o 0.1 % 0.05 H I 1 I- CM < FORSKOLIN cAMP IBMX PDGF O) CO jjUII 100 MICROMOLAR o 50 MICROMOLAR o CO O) | | 25 MICROMOLAR o a. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Supplementary Table S2
1-high in cerebrotropic Gene P-value patients Definition BCHE 2.00E-04 1 Butyrylcholinesterase PLCB2 2.00E-04 -1 Phospholipase C, beta 2 SF3B1 2.00E-04 -1 Splicing factor 3b, subunit 1 BCHE 0.00022 1 Butyrylcholinesterase ZNF721 0.00028 -1 Zinc finger protein 721 GNAI1 0.00044 1 Guanine nucleotide binding protein (G protein), alpha inhibiting activity polypeptide 1 GNAI1 0.00049 1 Guanine nucleotide binding protein (G protein), alpha inhibiting activity polypeptide 1 PDE1B 0.00069 -1 Phosphodiesterase 1B, calmodulin-dependent MCOLN2 0.00085 -1 Mucolipin 2 PGCP 0.00116 1 Plasma glutamate carboxypeptidase TMX4 0.00116 1 Thioredoxin-related transmembrane protein 4 C10orf11 0.00142 1 Chromosome 10 open reading frame 11 TRIM14 0.00156 -1 Tripartite motif-containing 14 APOBEC3D 0.00173 -1 Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3D ANXA6 0.00185 -1 Annexin A6 NOS3 0.00209 -1 Nitric oxide synthase 3 SELI 0.00209 -1 Selenoprotein I NYNRIN 0.0023 -1 NYN domain and retroviral integrase containing ANKFY1 0.00253 -1 Ankyrin repeat and FYVE domain containing 1 APOBEC3F 0.00278 -1 Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3F EBI2 0.00278 -1 Epstein-Barr virus induced gene 2 ETHE1 0.00278 1 Ethylmalonic encephalopathy 1 PDE7A 0.00278 -1 Phosphodiesterase 7A HLA-DOA 0.00305 -1 Major histocompatibility complex, class II, DO alpha SOX13 0.00305 1 SRY (sex determining region Y)-box 13 ABHD2 3.34E-03 1 Abhydrolase domain containing 2 MOCS2 0.00334 1 Molybdenum cofactor synthesis 2 TTLL6 0.00365 -1 Tubulin tyrosine ligase-like family, member 6 SHANK3 0.00394 -1 SH3 and multiple ankyrin repeat domains 3 ADCY4 0.004 -1 Adenylate cyclase 4 CD3D 0.004 -1 CD3d molecule, delta (CD3-TCR complex) (CD3D), transcript variant 1, mRNA. -

PDE7A, Active PDE7A, Active
Catalog # Aliquot Size P95-31G -05 5 µg P95-31G -10 10 µg PDE7A, Active Human recombinant protein expressed in Sf9 cells Catalog # P95-31G Lot # F723 -2 Product Description Specific Activity Recombinant human PDE7A (104-end) was expressed by baculovirus in Sf9 insect cells using an N-terminal GST tag. The gene accession number is NM_002603 . 2,400,000 Gene Aliases 1,800,000 1,200,000 HCP1; PDE7 600,000 Formulation (RLU) Activity 0 5 10 15 20 Recombinant protein stored in 50mM Tris-HCl, pH 7.5, 150mM NaCl, 10mM glutathione, 0.1mM EDTA, 0.25mM Protein (ng) DTT, 0.1mM PMSF, 25% glycerol. The specific activity of PDE7A was determined to be 160 nmol /min/mg as per activity assay protocol. Storage and Stability Purity o Store product at –70 C. For optimal storage, aliquot target into smaller quantities after centrifugation and store at recommended temperature. For most favorable performance, avoid repeated handling and multiple freeze/thaw cycles. The purity of PDE7A was Scientific Background determined to be >90% by densitometry. PDE7A is a member of the phosphodiesterase family of Approx. MW 64kDa . proteins that play a critical role in regulating intracellular levels of cAMP and cGMP. PDE7A is a high-affinity cAMP-specific PDE and is expressed in T cell lines, peripheral blood T lymphocytes, epithelial cell lines, airway and vascular smooth muscle cells, lung fibroblasts, and eosinophils. PDE7 plays a critical role in PDE7A, Active the regulation of human T cell function and selective Human recombinant protein expressed in Sf9 cells PDE7 inhibitors are being examined to treat immunological and inflammatory disorders (1). -

Oligonucleotide Compositions and Methods for Treating Disease Including Inflammatory Conditions
(19) & (11) EP 2 314 595 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 27.04.2011 Bulletin 2011/17 C07H 21/00 (2006.01) C12N 15/11 (2006.01) A61K 31/7088 (2006.01) A61P 29/00 (2006.01) (21) Application number: 10183977.7 (22) Date of filing: 29.09.2004 (84) Designated Contracting States: • Zemzoumi, Khalid AT BE BG CH CY CZ DE DK EE ES FI FR GB GR MONTREAL Québec H2H 1V3 (CA) HU IE IT LI LU MC NL PL PT RO SE SI SK TR • D’Anjou, Hélène BROSSARD Québec J4X 2W5 (CA) (30) Priority: 29.09.2003 US 507016 P (74) Representative: Cabinet Plasseraud (62) Document number(s) of the earlier application(s) in 52, rue de la Victoire accordance with Art. 76 EPC: 75440 Paris Cedex 09 (FR) 04786683.5 / 1 668 024 Remarks: (71) Applicant: Topigen Pharmaceuticals Inc. This application was filed on 30-09-2010 as a Montréal, QC H1W 4A4 (CA) divisional application to the application mentioned under INID code 62. (72) Inventors: • Renzi, Paolo WESTMOUNT Québec H3Y 1E9 (CA) (54) Oligonucleotide compositions and methods for treating disease including inflammatory conditions (57) The invention relates to therapeutic antisense structive pulmonary disease (COPD), acute respiratory oligonucleotides directed against genes coding for phos- distress syndrome, bronchitis, chronic bronchitis, silico- phodiesterase (PDEs) and the use of these in combina- sis, pulmonary fibrosis, lung allograft rejection, allergic tion. These antisense oligonucleotides may be used as rhinitis and chronic sinusitis as well as other conditions analytical tools and/or as therapeutic agents in the treat- in which an increase in cyclic AMP or a decrease in PDE ment of disease associated with reduced cellular cAMP levels is beneficial in a patient, such as inflammatory diseases of the respi- ratory tract including, for example, asthma, chronic ob- EP 2 314 595 A2 Printed by Jouve, 75001 PARIS (FR) EP 2 314 595 A2 Description Field of the Invention 5 [0001] The invention relates to methods, reagents and compositions of use for antisense oligonucleotide-based ther- apy. -

Harnessing the Power of P450 Enzymes Aaron T. Larsen
Harnessing the Power of P450 Enzymes A Chemical Auxiliary-Based Approach to Predictable P450 Oxidations at Inactivated C-H Bonds Aaron T. Larsen A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Doctor of Philosophy Department of Chemistry McGill University Montreal, Quebec, Canada H3A 2K6 Submitted: November, 2011 © Aaron T. Larsen, 2011 Abstract Abstract Enantioselective hydroxylation of one specific methylene in the presence of many similar groups is debatably the most challenging chemical transformation. Although chemists have recently made progress towards the hydroxylation of inactivated C-H bonds, enzymes like P450s (CYPs) remain unsurpassed in specificity and scope. The substrate promiscuity of many P450s is desirable for synthetic applications; however, the inability to predict the products of these enzymatic reactions and the poor activity and stability of these enzymes is impeding advancement. In chapter 2 of this thesis, we evaluate several strategies to improve the activity and stability of CYP3A4. These strategies include the immobilization of CYP3A4 inside molecular hydrogels and silica in addition to the chemical modification of CYP3A4 using various anhydrides. Although none of the strategies we investigate here greatly enhance the catalytic utility of the enzyme, CYP3A4 is shown to be highly tolerant to functionalization at a large number of surface residues and to the presence of extremely high concentrations of silica during catalysis. Recognizing the potential for enzymes containing small, hydrophobic active sites to catalyze Diels-Alder reactions, chapter 3 describes the design and application of several assays to evaluate the Diels-Alderase activity of CYP2E1. Although the presence of CYP2E1 is not found to increase the rates of the reactions we investigate here, the results do demonstrate that there is ii Abstract an interaction between one or more of the substrates and the enzyme at either the active site or at another binding pocket. -

Whole Genome Sequencing of Familial Non-Medullary Thyroid Cancer Identifies Germline Alterations in MAPK/ERK and PI3K/AKT Signaling Pathways
biomolecules Article Whole Genome Sequencing of Familial Non-Medullary Thyroid Cancer Identifies Germline Alterations in MAPK/ERK and PI3K/AKT Signaling Pathways Aayushi Srivastava 1,2,3,4 , Abhishek Kumar 1,5,6 , Sara Giangiobbe 1, Elena Bonora 7, Kari Hemminki 1, Asta Försti 1,2,3 and Obul Reddy Bandapalli 1,2,3,* 1 Division of Molecular Genetic Epidemiology, German Cancer Research Center (DKFZ), D-69120 Heidelberg, Germany; [email protected] (A.S.); [email protected] (A.K.); [email protected] (S.G.); [email protected] (K.H.); [email protected] (A.F.) 2 Hopp Children’s Cancer Center (KiTZ), D-69120 Heidelberg, Germany 3 Division of Pediatric Neurooncology, German Cancer Research Center (DKFZ), German Cancer Consortium (DKTK), D-69120 Heidelberg, Germany 4 Medical Faculty, Heidelberg University, D-69120 Heidelberg, Germany 5 Institute of Bioinformatics, International Technology Park, Bangalore 560066, India 6 Manipal Academy of Higher Education (MAHE), Manipal, Karnataka 576104, India 7 S.Orsola-Malphigi Hospital, Unit of Medical Genetics, 40138 Bologna, Italy; [email protected] * Correspondence: [email protected]; Tel.: +49-6221-42-1709 Received: 29 August 2019; Accepted: 10 October 2019; Published: 13 October 2019 Abstract: Evidence of familial inheritance in non-medullary thyroid cancer (NMTC) has accumulated over the last few decades. However, known variants account for a very small percentage of the genetic burden. Here, we focused on the identification of common pathways and networks enriched in NMTC families to better understand its pathogenesis with the final aim of identifying one novel high/moderate-penetrance germline predisposition variant segregating with the disease in each studied family. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Pentoxifylline As an Adjuvant Treatment in Renal Cell Carcinoma
Pentoxifylline As An Adjuvant Treatment In Renal Cell Carcinoma Item Type text; Electronic Dissertation Authors Mastrandrea, Nicholas Joseph Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 06/10/2021 16:37:11 Link to Item http://hdl.handle.net/10150/337293 PENTOXIFYLLINE AS AN ADJUVANT TREATMENT IN RENAL CELL CARCINOMA by Nicholas J. Mastrandrea A Dissertation Submitted to the Faculty of the DEPARTMENT OF PHARMACOLOGY AND TOXICOLOGY In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2014 1 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Nicholas J Mastrandrea, titled Pentoxifylline as an Adjuvant Treatment in Renal Cell Carcinoma and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy. ________________________________________________________ Date: 9/25/14 Serrine S. Lau, Ph. D. ________________________________________________________ Date: 9/25/14 Terrence J. Monks, Ph. D. ________________________________________________________ Date: 9/25/14 Donna Zhang, Ph. D. ________________________________________________________ Date: 9/25/14 Richard Vaillancourt, Ph. D. ________________________________________________________ Date: 9/25/14 George Tsaprailis, Ph. D. Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. I hereby certify that I have read this dissertation prepared under my direction and recommend that it be accepted as fulfilling the dissertation requirement.