Scenesse (Afamelanotide) NOTICE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulatory News
WHO Drug Information Vol. 28, No. 4, 2014 Regulatory news Ebola curative – transfusions of whole blood or blood plasma from recovered patients Update on treatments and vaccines have been scheduled to be conducted in Liberia, in line with WHO technical The Ebola crisis has prompted an guidelines (4). unprecedented cooperation between regulators In September the European Medicines to support WHO and to advise on possible Agency (EMA) established an expert pathways for the development, evaluation and group to review available information approval of medicines to fight Ebola. Progress on Ebola experimental treatments – towards provision of treatments and vaccines is excluding convalescent therapies – and summarized below. invited developers to submit their data (5). In August 2014, a WHO-convened panel Vaccines had agreed unanimously that is ethically On 29–30 September, 70 experts acceptable to use of experimental attended a WHO-convened consultation medicines and vaccines under the on Ebola vaccines. They took stock of the exceptional circumstances of the Ebola many ongoing efforts to rapidly evaluate epidemic (1). In early September, WHO the safety and efficacy of Ebola vaccines convened a consultation on potential for deployment as soon as possible to Ebola therapies and vaccines (2). The critical frontline workers and ultimately to importance of supportive care and populations at risk in mass vaccination community response was stressed in this campaigns. Two candidate vaccines have and subsequent discussions. clinical-grade vials available for safety trials. (6) Treatments In October, WHO convened industry In September, more than 200 experts leaders and key partners to discuss trials from around the world met at WHO and production of Ebola vaccine (7). -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Afamelanotide 16Mg Implant (SCENESSE) for Generalised Vitiligo – First Line in Combination with Narrow-Band Ultraviolet B Light (NB-UVB)
December Horizon Scanning Research & 2016 Intelligence Centre Afamelanotide 16mg Implant (SCENESSE) for generalised vitiligo – first line in combination with narrow-band ultraviolet B light (NB-UVB) NIHR HSRIC ID: 6667 Lay summary Afamelanotide is a new drug to treat generalised vitiligo. Vitiligo is a long-term condition where white patches develop on the skin, because of a lack of pigment in the skin. This condition may cause significant psychological distress. Afamelanotide is administered as an implant once every 28 days with NB-UVB light therapy administered 2-3 times per week. If licensed, afamelanotide may offer an additional treatment option for patients with this condition. This briefing is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement on the safety, efficacy or effectiveness of the health technology covered and should not be used for commercial purposes or commissioning without additional information. This briefing presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health. Horizon Scanning Research & Intelligence Centre University of Birmingham [email protected] www.hsric.nihr.ac.uk @OfficialNHSC TARGET GROUP • Generalised vitiligo: adults; stable or slowly progressing; with 10-50% of total body surface involvement – first line; in combination with narrow-band ultraviolet B light (NB- UVB). TECHNOLOGY DESCRIPTION Afamelanotide (SCENESSE; [Nle4,D-Phe7]-alpha-MSH; CUV-1647; EPT-1647; melanotan I; melanotan) is a structural analogue of endogenous alpha-melanocyte-stimulating hormone (α-MSH), which is formulated as a bioresorbable implant. -
Copyrighted Material
1 Index Note: Page numbers in italics refer to figures, those in bold refer to tables and boxes. References are to pages within chapters, thus 58.10 is page 10 of Chapter 58. A definition 87.2 congenital ichthyoses 65.38–9 differential diagnosis 90.62 A fibres 85.1, 85.2 dermatomyositis association 88.21 discoid lupus erythematosus occupational 90.56–9 α-adrenoceptor agonists 106.8 differential diagnosis 87.5 treatment 89.41 chemical origin 130.10–12 abacavir disease course 87.5 hand eczema treatment 39.18 clinical features 90.58 drug eruptions 31.18 drug-induced 87.4 hidradenitis suppurativa management definition 90.56 HLA allele association 12.5 endocrine disorder skin signs 149.10, 92.10 differential diagnosis 90.57 hypersensitivity 119.6 149.11 keratitis–ichthyosis–deafness syndrome epidemiology 90.58 pharmacological hypersensitivity 31.10– epidemiology 87.3 treatment 65.32 investigations 90.58–9 11 familial 87.4 keratoacanthoma treatment 142.36 management 90.59 ABCA12 gene mutations 65.7 familial partial lipodystrophy neutral lipid storage disease with papular elastorrhexis differential ABCC6 gene mutations 72.27, 72.30 association 74.2 ichthyosis treatment 65.33 diagnosis 96.30 ABCC11 gene mutations 94.16 generalized 87.4 pityriasis rubra pilaris treatment 36.5, penile 111.19 abdominal wall, lymphoedema 105.20–1 genital 111.27 36.6 photodynamic therapy 22.7 ABHD5 gene mutations 65.32 HIV infection 31.12 psoriasis pomade 90.17 abrasions, sports injuries 123.16 investigations 87.5 generalized pustular 35.37 prepubertal 90.59–64 Abrikossoff -

Kaiser Permanente Bernard J. Tyson School of Medicine, Inc. Exclusive Provider Organization (EPO) Student Blanket Health Plan Drug Formulary
Kaiser Permanente Bernard J. Tyson School of Medicine, Inc. Exclusive Provider Organization (EPO) Student Blanket Health Plan Drug Formulary Effective September 1, 2021 Health Plan Products: Kaiser Permanente Bernard J. Tyson School of Medicine, EPO Student Blanket Health Plan offered by Kaiser Permanente Insurance Company For the most current list of covered medications or for help understanding your KPIC insurance plan benefits, including cost sharing for drugs under the prescription drug benefit and under the medical benefit: Call 1-800-533-1833, TTY 711, Monday through Friday, 7 a.m. to 9 p.m. ET Visit kaiserpermanente.org to: • Find a participating retail pharmacy by ZIP code. • Look up possible lower-cost medication alternatives. • Compare medication pricing and options. • Find an electronic copy of the formulary here. • Get plan coverage information. For cost sharing information for the outpatient prescription drug benefits in your specific plan, please visit kp.org/kpic-websiteTBD The formulary is subject to change and all previous versions of the formulary are no longer in effect. Kaiser Permanente Last updated: September 1, 2021 Table of Contents Informational Section...........................................................................................................................................3 ANTIHISTAMINE DRUGS - Drugs for Allergy.....................................................................................................9 ANTI-INFECTIVE AGENTS - Drugs for Infections........................................................................................... -

WO 2016/195476 Al 8 December 2016 (08.12.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/195476 Al 8 December 2016 (08.12.2016) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/34 (2006.01) A61P 17/16 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/NL2015/050389 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 29 May 2015 (29.05.2015) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (71) Applicant: ERASMUS UNIVERSITY MEDICAL kind of regional protection available): ARIPO (BW, GH, CENTER ROTTERDAM [NL/NL]; Dr. Molewaterplein GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 50, NL-3015 GE Rotterdam (NL). TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (72) Inventors: SIJBRANDS, Eric Jacobus Gerardus; c/o Dr. -
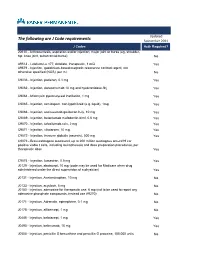
The Following Are J Code Requirements
Updated The following are J Code requirements September 2021 J Codes Auth Required? 20610 - Arthrocentesis, aspiration and/or injection; major joint or bursa (eg, shoulder, hip, knee joint, subacromial bursa) No A9513 - Lutetium Lu 177, dotatate, therapeutic, 1 mCi Yes A9579 - Injection, gadolinium-based magnetic resonance contrast agent, not otherwise specified (NOS), per ml No C9036 - Injection, patisiran, 0.1 mg Yes C9062 - Injection, daratumumab 10 mg and hyaluronidase-fihj Yes C9064 - Mitomycin pyelocalyceal instillation, 1 mg Yes C9065 - Injection, romidepsin, non-lypohilized (e.g. liquid), 1mg Yes C9066 - Injection, sacituzumab govitecan-hziy, 10 mg Yes C9069 - Injection, belantamab mafodontin-blmf, 0.5 mg Yes C9070 - Injection, tafasitamab-cxix, 2 mg Yes C9071 - Injection, viltolarsen, 10 mg Yes C9072 - Injection, immune globulin (asceniv), 500 mg Yes C9073 - Brexucabtagene autoleucel, up to 200 million autologous anti-cd19 car positive viable t cells, including leukapheresis and dose preparation procedures, per therapeutic dose Yes C9074 - Injection, lumasiran, 0.5 mg Yes J0129 - Injection, abatacept, 10 mg (code may be used for Medicare when drug administered under the direct supervision of a physician) Yes J0131 - Injection, Acetaminophen, 10 mg No J0133 - Injection, acyclovir, 5 mg No J0150 - Injection, adenosine for therapeutic use, 6 mg (not to be used to report any adenosine phosphate compounds, instead use A9270) No J0171 - Injection, Adrenalin, epinephrine, 0.1 mg No J0178 - Injection, aflibercept, 1 mg No J0485 - Injection, -

Anatomical Classification Guidelines V2021 EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2021
EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2021 Anatomical Classification Guidelines V2021 "The Anatomical Classification of Pharmaceutical Products has been developed and maintained by the European Pharmaceutical Marketing Research Association (EphMRA) and is therefore the intellectual property of this Association. EphMRA's Classification Committee prepares the guidelines for this classification system and takes care for new entries, changes and improvements in consultation with the product's manufacturer. The contents of the Anatomical Classification of Pharmaceutical Products remain the copyright to EphMRA. Permission for use need not be sought and no fee is required. We would appreciate, however, the acknowledgement of EphMRA Copyright in publications etc. Users of this classification system should keep in mind that Pharmaceutical markets can be segmented according to numerous criteria." © EphMRA 2021 Anatomical Classification Guidelines V2021 CONTENTS PAGE INTRODUCTION A ALIMENTARY TRACT AND METABOLISM 1 B BLOOD AND BLOOD FORMING ORGANS 28 C CARDIOVASCULAR SYSTEM 36 D DERMATOLOGICALS 51 G GENITO-URINARY SYSTEM AND SEX HORMONES 58 H SYSTEMIC HORMONAL PREPARATIONS (EXCLUDING SEX HORMONES) 68 J GENERAL ANTI-INFECTIVES SYSTEMIC 72 K HOSPITAL SOLUTIONS 88 L ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS 96 M MUSCULO-SKELETAL SYSTEM 106 N NERVOUS SYSTEM 111 P PARASITOLOGY 122 R RESPIRATORY SYSTEM 124 S SENSORY ORGANS 136 T DIAGNOSTIC AGENTS 143 V VARIOUS 145 Anatomical Classification Guidelines V2021 INTRODUCTION The Anatomical Classification was initiated in 1971 by EphMRA. It has been developed jointly by Intellus/PBIRG and EphMRA. It is a subjective method of grouping certain pharmaceutical products and does not represent any particular market, as would be the case with any other classification system. -

2020 Aetna Standard Plan
Plan for your best health Aetna Standard Plan Aetna.com Aetna is the brand name used for products and services provided by one or more of the Aetna group of subsidiary companies, including Aetna Life Insurance Company and its affiliates (Aetna). Aetna Pharmacy Management refers to an internal business unit of Aetna Health Management, LLC. Aetna Pharmacy Management administers, but does not offer, insure or otherwise underwrite the prescription drug benefits portion of your health plan and has no financial responsibility therefor. 2020 Pharmacy Drug Guide - Aetna Standard Plan Table of Contents INFORMATIONAL SECTION..................................................................................................................6 *ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS* - DRUGS FOR THE NERVOUS SYSTEM.................................................................................................................................16 *ALLERGENIC EXTRACTS/BIOLOGICALS MISC* - BIOLOGICAL AGENTS...............................18 *ALTERNATIVE MEDICINES* - VITAMINS AND MINERALS....................................................... 19 *AMEBICIDES* - DRUGS FOR INFECTIONS.....................................................................................19 *AMINOGLYCOSIDES* - DRUGS FOR INFECTIONS.......................................................................19 *ANALGESICS - ANTI-INFLAMMATORY* - DRUGS FOR PAIN AND FEVER............................19 *ANALGESICS - NONNARCOTIC* - DRUGS FOR PAIN AND FEVER......................................... -

Medical and Maintenance Treatments for Vitiligo
Medical and Maintenance Treatments for Vitiligo Thierry Passeron, MD, PhD KEYWORDS Vitiligo Medical treatments Topical steroids Calcineurin inhibitors Sytemic steroids Methotrexate KEY POINTS Medical treatments alone, or in combination with phototherapy, are key approaches for treating nonsegmental vitiligo and, to a lesser extent, for treating segmental vitiligo. The treatments can be useful for halting disease progression and have proved effective for inducing repigmentation and decreasing the risk of relapses. Although the treatments have some side effects and limitations, vitiligo often induces a marked decrease in the quality of life of affected individuals and in most cases the risk:benefit ratio is in favor of an active approach. Systemic and topical agents targeting the pathways involved in the loss of melanocytes and in the differentiation of melanocyte stem cells should provide even more effective approaches in the near future, thanks to the increased knowledge of the pathophysiology of vitiligo. INTRODUCTION complete depigmented lesions, however, and repigmentation may be difficult in lesions of There are 3 aims needed for the optimal care of some patients while their vitiligo remains inactive vitiligo patients: first, halting the disease progres- for years. Recent transcriptomic analysis showed sion; then, allowing complete repigmentation of an impaired Wnt signaling pathway in vitiligo le- lesional areas; and, finally, preventing relapses. sions preventing the differentiation of melanocyte There is still no therapeutic panacea for vitiligo stem cells.4 Fibroblasts of some areas, such as but current options can lead to significant hands and feet, produce Wnt inhibitors.5 This improvement of vitiligo lesions. Some areas, might contribute to a defect in melanocyte differ- such as the face, usually respond well to therapies entiation and could explain the difficulties for whereas they remain mostly ineffective for others, repigmenting those localizations. -

Lists of Medicinal Products for Rare Diseases in Europe*
March 2021 Lists of medicinal products for rare diseases in Europe* the www.orpha.net www.orphadata.org General Table of contents PART 1: List of orphan medicinal products in Europe with European orphan designation and European marketing authorization 3 Table of contents 3 Methodology 3 Classification by tradename 5 Annex 1: Orphan medicinal products withdrawn from the European Community Register of orphan medicinal products 22 Annex 2: Orphan medicinal products withdrawn from use in the European Union 31 Classification by date of MA in descending order 33 Classification by ATC category 34 Classification by MA holder 35 PART 2 : 37 List of medicinal products intended for rare diseases in Europe with European marketing authorization without an orphan designation in Europe 37 Table of contents 37 Methodology 37 Classification by tradename 38 Classification by date of MA in descending order 104 Classification by ATC category 106 Classification by MA holder 108 For any questions or comments, please contact us: [email protected] Orphanet Report Series - Lists of medicinal products for rare diseases in Europe. March 2021 http://www.orpha.net/orphacom/cahiers/docs/GB/list_of_orphan_drugs_in_europe.pdf 2 PART 1: List of orphan medicinal products in Europe with European orphan designation and European marketing authorization* Table of contents List of orphan medicinal products in Europe with European orphan designation and European marketing authorisation* 3 Methodology 3 Classification by tradename 5 Annex 1: Orphan medicinal products removed or withdrawn from the European Community Register of orphan medicinal products 22 Annex 2: Orphan medicinal products withdrawn from use in the European Union 31 Classification by date of MA in descending order 33 Classification by ATC category 34 Classification by MA holder 35 Methodology This part of the document provides the list of all orphan with the list of medicinal products that have been granted a medicinal products that have received a European Marketing marketing authorization (http://ec.europa. -

Resolution Pharmacology Therapeutic Innovation in Inflammation.ACTH: the Forgotten Therapy Trinidad Montero-Melendez the William
Resolution Pharmacology therapeutic innovation in inflammation.ACTH: The Forgotten Therapy Trinidad Montero-Melendez The William Harvey Research Institute, Barts and The London School of Medicine, Queen Mary University of London. Charterhouse Square, EC1M 6BQ, London, United Kingdom. Correspondence: Trinidad Montero-Melendez, PhD Address: The William Harvey Research Institute, Barts and The London School of Medicine, Charterhouse Square, EC1M 6BQ, London, United Kingdom. Telephone: +44-207-8825654 Fax: +44-207-8826076 E-mail: [email protected] Abstract Although anti-inflammatory drugs are among the most common class of marketed drugs, chronic inflammatory conditions such as rheumatoid arthritis, multiple sclerosis or inflammatory bowel disease still represent unmet needs. New first-in-class drugs might be discovered in the future but the repurpose and further development of old drugs also offers promise for these conditions. This is the case of the melanocortin adrenocorticotropin hormone, ACTH, used in patients since 1952 but regarded as the last therapeutic option when other medications, such as glucocorticoids, cannot be used. Better understanding on its physiological and pharmacological mechanisms of actions and new insights on melanocortin receptors biology have revived the interest on rescuing this old and effective drug. ACTH does not only induce cortisol production, as previously assumed, but it also exerts anti-inflammatory actions by targeting melanocortin receptors present on immune cells. The endogenous agonists for these receptors (ACTH, α-, β-, and γ-melanocyte stimulating hormones), are also produced locally by immune cells, indicating the existence of an endogenous anti-inflammatory tissue-protective circuit involving the melanocortin system. These findings suggested that new ACTH-like melanocortin drugs devoid of steroidogenic actions, and hence side effects, could be developed.