Forest Insect and Disease History of the Carson National Forest - DRAFT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Building 27, Suite 3 Fort Missoula Road Missoula, MT 59804
Photo by Louis Kamler. www.nationalforests.org Building 27, Suite 3 Fort Missoula Road Missoula, MT 59804 Printed on recycled paper 2013 ANNUAL REPORT Island Lake, Eldorado National Forest Desolation Wilderness. Photo by Adam Braziel. 1 We are pleased to present the National Forest Foundation’s (NFF) Annual Report for Fiscal Year 2013. During this fourth year of the Treasured Landscapes campaign, we have reached $86 million in both public and private support towards our $100 million campaign goal. In this year’s report, you can read about the National Forests comprising the centerpieces of our work. While these landscapes merit special attention, they are really emblematic of the entire National Forest System consisting of 155 National Forests and 20 National Grasslands. he historical context for these diverse and beautiful Working to protect all of these treasured landscapes, landscapes is truly inspirational. The century-old to ensure that they are maintained to provide renewable vision to put forests in a public trust to secure their resources and high quality recreation experiences, is National Forest Foundation 2013 Annual Report values for the future was an effort so bold in the late at the core of the NFF’s mission. Adding value to the 1800’s and early 1900’s that today it seems almost mission of our principal partner, the Forest Service, is impossible to imagine. While vestiges of past resistance what motivates and challenges the NFF Board and staff. to the public lands concept live on in the present, Connecting people and places reflects our organizational the American public today overwhelmingly supports values and gives us a sense of pride in telling the NFF maintaining these lands and waters in public ownership story of success to those who generously support for the benefit of all. -

IMBCR Report
Integrated Monitoring in Bird Conservation Regions (IMBCR): 2015 Field Season Report June 2016 Bird Conservancy of the Rockies 14500 Lark Bunting Lane Brighton, CO 80603 303-659-4348 www.birdconservancy.org Tech. Report # SC-IMBCR-06 Bird Conservancy of the Rockies Connecting people, birds and land Mission: Conserving birds and their habitats through science, education and land stewardship Vision: Native bird populations are sustained in healthy ecosystems Bird Conservancy of the Rockies conserves birds and their habitats through an integrated approach of science, education and land stewardship. Our work radiates from the Rockies to the Great Plains, Mexico and beyond. Our mission is advanced through sound science, achieved through empowering people, realized through stewardship and sustained through partnerships. Together, we are improving native bird populations, the land and the lives of people. Core Values: 1. Science provides the foundation for effective bird conservation. 2. Education is critical to the success of bird conservation. 3. Stewardship of birds and their habitats is a shared responsibility. Goals: 1. Guide conservation action where it is needed most by conducting scientifically rigorous monitoring and research on birds and their habitats within the context of their full annual cycle. 2. Inspire conservation action in people by developing relationships through community outreach and science-based, experiential education programs. 3. Contribute to bird population viability and help sustain working lands by partnering with landowners and managers to enhance wildlife habitat. 4. Promote conservation and inform land management decisions by disseminating scientific knowledge and developing tools and recommendations. Suggested Citation: White, C. M., M. F. McLaren, N. J. -

Status and Ecology of Mexican Spotted Owls in the Upper Gila Mountains Recovery Unit, Arizona and New Mexico
Status and Ecology of Mexican Spotted Owls in the Upper Gila Mountains Recovery Unit, Arizona and New Mexico Joseph L. Ganey James P. Ward, Jr. David W. Willey United States Forest Rocky Mountain General Technical Report Department Service Research Station RMRS-GTR-256WWW of Agriculture May 2011 Ganey, Joseph L.; Ward, James P. Jr.; Willey, David W. 2011. Status and ecology of Mexican spotted owls in the Upper Gila Mountains recovery unit, Arizona and New Mexico. Gen. Tech. Rep. RMRS-GTR-256WWW. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 94 p. Abstract This report summarizes current knowledge on the status and ecology of the Mexican spot- ted owl within the Upper Gila Mountains Recovery Unit (UGM RU). It was written at the request of U.S. Forest Service personnel involved in the Four Forests Restoration Initia- tive (4FRI), a collaborative, landscape-scale restoration effort covering approximately 2.4 million ac (1 million ha) across all or part of four National Forests (Apache-Sitgreaves, Coconino, Kaibab, and Tonto National Forests) located within the UGM RU. The UGM RU supports >50% of the known population of Mexican spotted owls, and the central location of the UGM RU within the overall range of the owl appears to facilitate gene flow throughout that range. Consequently, the UGM population is viewed as important to stability within the overall range of the owl, and management that impacts owls within the UGM RU could affect owl populations beyond that RU. Keywords: abundance, demography, habitat selection, diet composition, movements Authors Joseph L. -

Lincoln National Forest
Chapter 1: Introduction In Ecological and Biological Diversity of National Forests in Region 3 Bruce Vander Lee, Ruth Smith, and Joanna Bate The Nature Conservancy EXECUTIVE SUMMARY We summarized existing regional-scale biological and ecological assessment information from Arizona and New Mexico for use in the development of Forest Plans for the eleven National Forests in USDA Forest Service Region 3 (Region 3). Under the current Planning Rule, Forest Plans are to be strategic documents focusing on ecological, economic, and social sustainability. In addition, Region 3 has identified restoration of the functionality of fire-adapted systems as a central priority to address forest health issues. Assessments were selected for inclusion in this report based on (1) relevance to Forest Planning needs with emphasis on the need to address ecosystem diversity and ecological sustainability, (2) suitability to address restoration of Region 3’s major vegetation systems, and (3) suitability to address ecological conditions at regional scales. We identified five assessments that addressed the distribution and current condition of ecological and biological diversity within Region 3. We summarized each of these assessments to highlight important ecological resources that exist on National Forests in Arizona and New Mexico: • Extent and distribution of potential natural vegetation types in Arizona and New Mexico • Distribution and condition of low-elevation grasslands in Arizona • Distribution of stream reaches with native fish occurrences in Arizona • Species richness and conservation status attributes for all species on National Forests in Arizona and New Mexico • Identification of priority areas for biodiversity conservation from Ecoregional Assessments from Arizona and New Mexico Analyses of available assessments were completed across all management jurisdictions for Arizona and New Mexico, providing a regional context to illustrate the biological and ecological importance of National Forests in Region 3. -

Assessment Report of Ecological / Social / Economic Conditions, Trends, and Risks to Sustainability, Cibola National Forest Mountain Ranger Districts
United States Department of Agriculture Forest Service Southwestern Region April 2014 Assessment Report of Ecological / Social / Economic Conditions, Trends, and Risks to Sustainability, Cibola National Forest Mountain Ranger Districts Cibola National Forest Mountain Ranger Districts Assessment Literature Cited Prepared by: The Cibola National Forest and Grasslands 2113 Osuna Rd., NE Albuquerque, NM 87113 For further information, contact: Elaine Kohrman Forest Supervisor Cibola National Forest and Grasslands 505-346-3900 ABSTRACT: The Assessment presents and evaluates existing information about relevant ecological, economic, and social conditions, trends, and risks to sustainability and their relationship to the 1985 Cibola Forest Plan, within the context of the broader landscape. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, sexual orientation, marital status, family status, status as a parent (in education and training programs and activities), because all or part of an individual’s income is derived from any public assistance program, or retaliation. (Not all prohibited bases apply to all programs or activities.) If you require this information in alternative format (Braille, large print, audiotape, etc.), contact the USDA’s TARGET Center at (202) 720-2600 (Voice or TDD). If you require information about this program, activity, or facility in a language other than English, contact the agency office responsible for the program or activity, or any USDA office. To file a complaint alleging discrimination, write USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call toll free, (866) 632-9992 (Voice). -

Insects That Feed on Trees and Shrubs
INSECTS THAT FEED ON COLORADO TREES AND SHRUBS1 Whitney Cranshaw David Leatherman Boris Kondratieff Bulletin 506A TABLE OF CONTENTS DEFOLIATORS .................................................... 8 Leaf Feeding Caterpillars .............................................. 8 Cecropia Moth ................................................ 8 Polyphemus Moth ............................................. 9 Nevada Buck Moth ............................................. 9 Pandora Moth ............................................... 10 Io Moth .................................................... 10 Fall Webworm ............................................... 11 Tiger Moth ................................................. 12 American Dagger Moth ......................................... 13 Redhumped Caterpillar ......................................... 13 Achemon Sphinx ............................................. 14 Table 1. Common sphinx moths of Colorado .......................... 14 Douglas-fir Tussock Moth ....................................... 15 1. Whitney Cranshaw, Colorado State University Cooperative Extension etnomologist and associate professor, entomology; David Leatherman, entomologist, Colorado State Forest Service; Boris Kondratieff, associate professor, entomology. 8/93. ©Colorado State University Cooperative Extension. 1994. For more information, contact your county Cooperative Extension office. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, -

Cibola National Forest Land Management Plan Revision Draft Environmental Impact Statement
United States Department of Agriculture Cibola National Forest Land Management Plan Revision Draft Environmental Impact Statement Forest Service Cibola National Forest and National Grasslands Mountain Ranger Districts MB-R3-03-29 August 2018 In accordance with Federal civil rights law and U.S. Department of Agriculture (USDA) civil rights regulations and policies, the USDA, its Agencies, offices, and employees, and institutions participating in or administering USDA programs are prohibited from discriminating based on race, color, national origin, religion, sex, gender identity (including gender expression), sexual orientation, disability, age, marital status, family/parental status, income derived from a public assistance program, political beliefs, or reprisal or retaliation for prior civil rights activity, in any program or activity conducted or funded by USDA (not all bases apply to all programs). Remedies and complaint filing deadlines vary by program or incident. Persons with disabilities who require alternative means of communication for program information (e.g., Braille, large print, audiotape, American Sign Language, etc.) should contact the responsible Agency or USDA’s TARGET Center at (202) 720-2600 (voice and TTY) or contact USDA through the Federal Relay Service at (800) 877-8339. Additionally, program information may be made available in languages other than English. To file a program discrimination complaint, complete the USDA Program Discrimination Complaint Form, AD- 3027, found online at Filing a USDA Program Discrimination Complaint and at any USDA office or write a letter addressed to USDA and provide in the letter all of the information requested in the form. To request a copy of the complaint form, call (866) 632-9992. -

Kiowa and Rita Blanca National Grasslands Travel Management Environmental Assessment
Kiowa and Rita Blanca National Grasslands Travel Management Environmental Assessment Recreation Specialist Report (Unit K-109 accessed by National Forest System Road K107, Harding County, New Mexico) Prepared by: John G. Baumchen Recreation Specialist for: Kiowa and Rita Blanca National Grasslands Cibola National Forest February 6th, 2012 Background The Kiowa and Rita Blanca Ranger District is comprised of two National Grasslands: The Kiowa National Grasslands (NG), which covers 137,157 acres and is located within Mora, Harding, Union, and Colfax Counties, New Mexico, while the Rita Blanca NG, which covers 92,989 acres located in Dallam County, Texas and in Cimarron County, Oklahoma The district office is located in Clayton, New Mexico. It is just west of the eastern portion of the Kiowa, while the villages of Roy and Mosquero, New Mexico are south of the western part of the Kiowa. The small unincorporated community of Felt, Oklahoma is within the Rita Blanca NG. Texline, Texas is along the southwest boundary of the Rita Blanca. Dalhart, Texas is south of the Rita Blanca while Stratford, Texas is just east of the Rita Blanca. The district is located in the southern portion of the North American Great Plains region in the short grass prairie. It is located in a sparsely-populated rural area, that is away from population centers, is isolated, and only has a few developed recreational facilities. The district receives a low to moderate amount of motor vehicle use related to recreational activities. There are several larger communities in the three-state region near the district office, including: Raton, New Mexico, approximately 83 miles to the northwest, Guymon, Oklahoma, approximately 105 miles to the east, Tucumcari, New Mexico, about 112 miles to the southwest, Amarillo, Texas, about 131 miles southeast, and Las Vegas, New Mexico, about 150 miles to the southwest. -

1985 Land and Resource Management Plan
Cibola National Forest Land and Resource Management Plan Table of Contents Page 1. INTRODUCTION Purpose of the Plan . 1 Relationship to Other Planning Levels and Studies . 1 Planning Process. 2 Organization of the Proposed Forest Plan Document . 5-1 Planning Area Description . 5-1 2. PUBLIC ISSUES AND MANAGEMENT CONCERNS Overview. 7 Firewood and Miscellaneous Products . 7 Range Management. 7 Soil and Water. 8 Recreation. 8 Mineral’s Management. 9 Transportation. 9 Electronic Site Management. 10 Wilderness Management . 10 Riparian Management . 10 Unauthorized Use. 11 National Grasslands . 11 Public Information and Education. 11 3. SUMMARY OF THE ANALYSIS OF THE MANAGEMENT SITUATION Overview. 13 Timber and Firewood . 14 Wilderness. 16 Wildlife and Fish . 17 Range . 19 Recreation. 20 Minerals. 22 Soil and Water. 24 Cultural Resources. 24 Research Natural Areas. 25 Diversity . 26 Visual Resources. 26 Lands and Special Uses. 27 Listed Wild, Scenic and Recreational Rivers . 28 Air . 28 Protection. 28 Facilities. 30 4. MANAGEMENT DIRECTION Mission . 33 Goals . 33 Objectives. 34 Management Prescriptions. 54 Management Prescriptions Applicable to all Areas. 56 Management Area 1 (Sandia Mountain Wilderness). 81 Management Area 2 (Sandia Ranger District). 84 Management Area 3 (Manzano Mountain, Apache Kid, and Withington Wildernesses) . 95 Management Area 4 (Black Kettle and McClellan Creek National Grasslands). 99 Management Area 5 (Kiowa and Rita Blanca National Grasslands) . 105 Management Area 7 (Langmuir Research site) . 109 Management Area 8 (Mt. Taylor Ranger District). 117 Management Area 9 (Mt. Taylor Ranger District). 127 Management Area 10 (Mt. Talyor Ranger District) . 133 Management Area 11 (Magdalena and Mountainair Ranger Districts) . 141 Management Area 12 (Mountainair and Magdalena Ranger Districts) . -

Carson National Forest Fact Sheet
Carson National Forest Fact Sheet 208 Cruz Alta Road Taos New Mexico 87571 (575) 758-6200 Geography: How many acres comprise the Carson National Forest? 1.5 million acres How many acres comprise Wheeler Peak Wilderness and what is the elevation of Wheeler Peak? 19,000 acres and 13,161 feet and is part of the Sangre do Cristo Mountains, Southern part of the Rockies. How many acres comprise Latir Peak Wilderness? 20,000 acres How many acres comprise the Cruses Basin Wilderness? 19,000 acres How many acres comprise Pecos Wilderness on the Carson National Forest? 24,735 acres What is the elevation of Picuris Peak? 10,801 feet What is the elevation of Pueblo Peak? 12,305 feet Hiking Information: How many miles of trails are on the Carson National Forest? Approximately 330 miles What is the easiest and closest hiking trail near Taos? Devisadero Loop (Trail #108), 5 mile loop, Located 3 miles from Taos on Highway 64 East. What are the most asked about trails on the Carson National Forest? Wheeler Peak #67 and Williams Lake Trail #62 What should I do in order to be prepared to hike Wheeler Peak? Wheeler Peak is the highest peak in New Mexico (13,161 feet), it is best to be acclimated to high altitude (hiking above tree line and along exposed ridges). Weather can change rapidly, especially during the summer monsoon months (late June to early August). Make sure to take plenty of water, raingear and wear layers of clothing. Camping: Where can I disperse camp on the Carson National Forest? Dispersed camping can be anywhere in the Carson National Forest, 100 feet from river/streams, roads and trails for a maximum of 14 days. -
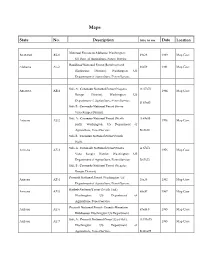
State No. Description Size in Cm Date Location
Maps State No. Description Size in cm Date Location National Forests in Alabama. Washington: ALABAMA AL-1 49x28 1989 Map Case US Dept. of Agriculture, Forest Service. Bankhead National Forest (Bankhead and Alabama AL-2 66x59 1981 Map Case Blackwater Districts). Washington: US Department of Agriculture, Forest Service. Side A : Coronado National Forest (Nogales A: 67x72 ARIZONA AZ-1 1984 Map Case Ranger District). Washington: US Department of Agriculture, Forest Service. B: 67x63 Side B : Coronado National Forest (Sierra Vista Ranger District). Side A : Coconino National Forest (North A:69x88 Arizona AZ-2 1976 Map Case Half). Washington: US Department of Agriculture, Forest Service. B:69x92 Side B : Coconino National Forest (South Half). Side A : Coronado National Forest (Sierra A:67x72 Arizona AZ-3 1976 Map Case Vista Ranger District. Washington: US Department of Agriculture, Forest Service. B:67x72 Side B : Coronado National Forest (Nogales Ranger District). Prescott National Forest. Washington: US Arizona AZ-4 28x28 1992 Map Case Department of Agriculture, Forest Service. Kaibab National Forest (North Unit). Arizona AZ-5 68x97 1967 Map Case Washington: US Department of Agriculture, Forest Service. Prescott National Forest- Granite Mountain Arizona AZ-6 67x48.5 1993 Map Case Wilderness. Washington: US Department of Agriculture, Forest Service. Side A : Prescott National Forest (East Half). A:111x75 Arizona AZ-7 1993 Map Case Washington: US Department of Agriculture, Forest Service. B:111x75 Side B : Prescott National Forest (West Half). Arizona AZ-8 Superstition Wilderness: Tonto National 55.5x78.5 1994 Map Case Forest. Washington: US Department of Agriculture, Forest Service. Arizona AZ-9 Kaibab National Forest, Gila and Salt River 80x96 1994 Map Case Meridian. -

RECREATIONAL FEE DEMONSTRATION PROGRAM Progress Report to Congress Fiscal Year 2000
RECREATIONAL FEE DEMONSTRATION PROGRAM Progress Report to Congress Fiscal Year 2000 Submitted by the U.S. Department of the Interior National Park Service U.S. Fish and Wildlife Service Bureau of Land Management U.S. Department of Agriculture Forest Service January 31, 2001 Table of Contents Executive Summary ............................................................iii I. Background and Data ......................................................... 1 A. Background ......................................................... 1 B. Recreation Visits ..................................................... 2 Table 1. Number of Recreation Visitors ................................ 3 C. Recreation Fee Revenues ............................................... 4 Table 2. Gross Revenues ............................................ 5 D. Cost of Collecting Recreation Fees ....................................... 6 Table 3. Cost of Fee Collection ...................................... 7 E. Obligation of Fee Demonstration Revenues ................................. 8 Table 4. Disposition of Revenues ..................................... 9 Table 5. Department of the Interior Obligations by Category ............... 10 Table 6. National Park Service Obligations by Category .................. 11 Table 7. U.S. Fish and Wildlife Service Obligations by Category ........... 12 Table 8. Bureau of Land Management Obligations by Category ............ 13 Table 9. USDA Forest Service Obligations by Category .................. 14 II. Accomplishments of the Program ............................................