Ground Water Quality Scenario in India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Final Electoral Roll
FINAL ELECTORAL ROLL - 2018 STATE - (S24) UTTAR PRADESH No., Name and Reservation Status of Assembly Constituency: 291-Tulsipur(GEN) Last Part No., Name and Reservation Status of Parliamentary Service Constituency in which the Assembly Constituency is located: 58-Shrawasti(GEN) Electors 1. DETAILS OF REVISION Year of Revision : 2018 Type of Revision : Summary Revision Qualifying Date : 01.01.2018 Date of Final Publication: 31.01.2018 2. SUMMARY OF SERVICE ELECTORS A) NUMBER OF ELECTORS 1. Classified by Type of Service Name of Service No. of Electors Members Wives Total A) Defence Services 25 1 26 B) Armed Police Force 0 0 0 C) Foreign Service 1 0 1 Total in Part (A+B+C) 26 1 27 2. Classified by Type of Roll Roll Type Roll Identification No. of Electors Members Wives Total I Original Mother roll, Summary Revision of Last Part 26 1 27 2017 of Electoral Roll, 2018 II Additions Supplement 1 After Draft publication, 2018 0 0 0 List Sub Total: 0 0 0 III Deletions Supplement 1 After Draft publication, 2018 0 0 0 List Sub Total: 0 0 0 Net Electors in the Roll after (I + II - III) 26 1 27 B) NUMBER OF CORRECTIONS/MODIFICATION Roll Type Roll Identification No. of Electors Supplement 1 After Draft publication, 2018 0 Total: 0 Elector Type: M = Member, W = Wife Page 1 Final Electoral Roll, 2018 of Assembly Constituency 291-Tulsipur (GEN), (S24) UTTAR PRADESH A . Defence Services Sl.No Name of Elector Elector Rank Husband's Address of Record House Address Type Sl.No. Officer/Commanding Officer for despatch of Ballot Paper (1) (2) (3) (4) (5) (6) (7) -

Brief Industrial Profile of Bhojpur District
Government of India Ministry of MSME Brief Industrial Profile of Bhojpur District Carried out by MSME -Development Institute (Ministry of MSME, Govt. of India,) Patliputra Industrial Estate, PATNA - 800013 Phone: 0612-2262186/2262208/2262719 Fax: 0612=2262186 e-mail: [email protected] Web-www.msmedipatna.gov.in 1 Contents S. No. Topic Page No. 1. General Characteristics of the District 03 1.1 Location & Geographical Area 03 1.2 Topography 03 1.3 Availability of Minerals. 03 1.4 Forest 04 1.5 Administrative set up 04 2. District at a glance 04 2.1 Existing Status of Industrial Area in the District Bhojpur 07 3. Industrial Scenario Of Bhojpur 07 3.1 Industry at a Glance 07 3.2 Year Wise Trend Of Units Registered 08 3.3 Details Of Existing Micro & Small Enterprises & Artisan Units In The 09 District 3.4 Large Scale Industries / Public Sector undertakings 09 3.5 Major Exportable Item 09 3.6 Growth Trend 09 3.7 Vendorisation / Ancillarisation of the Industry 10 3.8 Medium Scale Enterprises 10 3.8.1 List of the units in Bhojpur & near by Area 10 3.8.2 Major Exportable Item 10 3.9 Service Enterprises 10 3.9.1 Coaching Industry == 3.9.2 Potentials areas for service industry 10 3.10 Potential for new MSMEs 10 4. Existing Clusters of Micro & Small Enterprise 10 4.1 Detail Of Major Clusters 10 4.1.1 Manufacturing Sector 10 4.1.2 Service Sector 11 4.2 Details of Identified cluster 11 5. General issues raised by industry association during the course of 12 meeting 6 Steps to set up MSMEs 13 2 Brief Industrial Profile of Bhojpur District 1. -

Of India 100935 Parampara Foundation Hanumant Nagar ,Ward No
AO AO Name Address Block District Mobile Email Code Number 97634 Chandra Rekha Shivpuri Shiv Mandir Road Ward No 09 Araria Araria 9661056042 [email protected] Development Foundation Araria Araria 97500 Divya Dristi Bharat Divya Dristi Bharat Chitragupt Araria Araria 9304004533 [email protected] Nagar,Ward No-21,Near Subhash Stadium,Araria 854311 Bihar Araria 100340 Maxwell Computer Centre Hanumant Nagar, Ward No 15, Ashram Araria Araria 9934606071 [email protected] Road Araria 98667 National Harmony Work & Hanumant Nagar, Ward No.-15, Po+Ps- Araria Araria 9973299101 [email protected] Welfare Development Araria, Bihar Araria Organisation Of India 100935 Parampara Foundation Hanumant Nagar ,Ward No. 16,Near Araria Araria 7644088124 [email protected] Durga Mandir Araria 97613 Sarthak Foundation C/O - Taranand Mishra , Shivpuri Ward Araria Araria 8757872102 [email protected] No. 09 P.O + P.S - Araria Araria 98590 Vivekanand Institute Of 1st Floor Milan Market Infront Of Canara Araria Araria 9955312121 [email protected] Information Technology Bank Near Adb Chowk Bus Stand Road Araria Araria 100610 Ambedkar Seva Sansthan, Joyprakashnagar Wardno-7 Shivpuri Araria Araria 8863024705 [email protected] C/O-Krishnamaya Institute Joyprakash Nagar Ward No -7 Araria Of Higher Education 99468 Prerna Society Of Khajuri Bazar Araria Bharga Araria 7835050423 [email protected] Technical Education And ma Research 100101 Youth Forum Forbesganj Bharga Araria 7764868759 [email protected] -

State District Name of Bank Bank Branch/ Financial Literacy Centre
State District Name of Bank Branch/ Address ITI Code ITI Name ITI Address State District Phone Email Bank Financial Category Number Literacy Centre Bihar Araria State Araria Lead Bank Office, PR10000055 Al-Sahaba Industrial P Alamtala Forbesganj Bihar Araria NULL Bank of ADB Building, Training Institute India Araria, Pin- 854311 Bihar Arwal PNB ARWAL ARWAL PR10000083 Adarsh ITC P Umerabad Bihar Arwal NULL Bihar Arwal PNB ARWAL ARWAL PR10000284 Shakuntalam ITC P Prasadi English Bihar Arwal NULL Bihar Arwal PNB ARWAL ARWAL PR10000346 Aditya ITC P At. Wasilpur, Main Road, Bihar Arwal NULL P.O. Arwal, Bihar Arwal PNB ARWAL ARWAL PR10000396 Vikramshila Private P At. Rojapar, P.O. Arwal Bihar Arwal NULL ITI Bihar Arwal PNB ARWAL ARWAL PR10000652 Ram Bhaman Singh P At-Purani Bazar P.o+P.S- Bihar Arwal NULL Private ITI Arwal Bihar Arwal PNB ARWAL ARWAL PR10000677 Sukhdeo Institute Of P Kurtha, Arwal Bihar Arwal NULL Tecnology Private ITI, Bihar Arwal PNB ARWAL ARWAL PR10000707 Dr. Rajendra Prasad P Mubarkpur, Kurtha Arwal Bihar Arwal NULL Private ITI, Bihar Aurangabad PUNJAB DAUDNAGAR DAUDNAGAR PR10000027 New Sai Private ITI- P Aurangabad Road, Bihar Aurangabad NULL NATIONA Bhakharuan More, , Tehsil- L BANK Daudnagar , , Aurangabad - 824113 Bihar Aurangabad PUNJAB AURANGABAD AURANGABAD PR10000064 Adharsh Industrial P Josai More Udyog Bihar Aurangabad NULL NATIONA Training Centre Pradhikar Campus L BANK Bihar Aurangabad MADHYA DAUDNAGAR DAUDNAGAR PR10000108 Sardar Vallabh Bhai P Daudnagar Bihar Aurangabad NULL BIHAR Patel ITC, Daudnagar GRAMIN BANK Bihar Aurangabad MADHYA DAUDNAGAR DAUDNAGAR PR10000142 Adarsh ITC, P AT-,Growth centre ,Jasoia Bihar Aurangabad NULL BIHAR Daudnagar More Daudnagar GRAMIN BANK Bihar Aurangabad PUNJAB RATANUA RATANUA PR10000196 Progresive ITC P At-Growth Center Josia Bihar Aurangabad NULL NATIONA More L BANK Bihar Aurangabad MADHYA DAUDNAGAR DAUDNAGAR PR10000199 Arya Bhatt ITC P Patel Nagar, Daud Nagar Bihar Aurangabad NULL BIHAR GRAMIN BANK Bihar Aurangabad PUNJAB OLD GT RD. -

Bhojpur District, Bihar State
भूजल सूचना पुस्तिका भोजपुर स्जला, बिहार Ground Water Information Booklet Bhojpur District, Bihar State 84° 15' 84° 30' 84° 45' BIHAR STATE ADMINISTRATIVE MAP N BHOJPUR DISTRICT, BIHAR. Ganga R. 25° 25° 45' 45' GANGA RIVER Barhara Gaura Bhagar N. Dehra N. Shahpur Gangi N. Koilwar Chher N. Bihiya ARA 25° Udawant 25° nagar 30' Gangi N. 30' Jagdishpur Garhani KumhariSandes N. Charpokhari Agiaon Banas N. Piro 25° Sahar 25° 15' SON RIVER 15' LEGEND Tarari District Boundary Block Boundary River District HQ. 0 5 10 15 20 Km Block HQ. Road Scale Railway 84° 15' 84° 30' 84° 45' के न्द्रीय भमू िजल िो셍 ड Central Ground water Board Ministry of Water Resources जल संसाधन िंत्रालय (Govt. of India) (भारि सरकार) Mid-Eastern Region िध्य-पर्वू ी क्षेत्र Patna पटना मसिंिर 2013 September 2013 1 PREPARED BY - Shri S. Sahu Sc. B UNDER SUPERVISION OF - Shri A.K.Agrawal, Scientist’D’ CARTOGRAPHY - Shri Lokendra Kumar, D/Man Gd-II 2 UPDATED BY - Shri S.N.Dwivedi, Sc- C & Dr. Fakhre Alam, STA (Hg) CONTENTS DISTRICT AT A GLANCE. 5-6 1. INTRODUCTION 7 - 10 1.1 Location, Area and Administrative Details 1.2 Basin/Sub-Basin and Drainage 1.3 Landuse, Agriculture and Irrigation Practices 1.4 Studies/Activities carried by CGWB 2. CLIMATE AND RAINFALL 10 3. GEOMORPHOLOGY AND SOIL 10-11 3.1 Geomorphology 3.2 Soil 4. GROUND WATER SCENARIO 11 - 17 4.1 Water Bearing Formations 4.2 Occurrence & Movement of Ground Water 4.3 Depth to Water Level 4.4 Ground Water Quality 4.4.1 Arsenic in Ground Water 4.5 Ground Water Resources 5. -

Bhojpur 2019-20
Ministry of Micro, Small & Medium Enterprises Government of India DISTRICT PROFILE BHOJPUR 2019-20 Carried out by MSME-Development Institute (Ministry of MSME, Govt. of India,) Patliputra Industrial Estate, Patna-13 Phone:- 0612-2262719, 2262208, 2263211 Fax: 06121 -2262186 e-mail: [email protected] Web- www.msmedipatna.gov.in Veer Kunwar Singh Memorial, Ara, Bhojpur Sun Temple, Tarari, Bhojpur 2 FOREWORD At the instance of the Development Commissioner, Micro, Small & Medium Enterprises, Government of India, New Delhi, District Industrial Profile containing basic information about the district of Bhojpur has been updated by MSME-DI, Patna under the Annual Plan 2019-20. It covers the information pertaining to the availability of resources, infrastructural support, existing status of industries, institutional support for MSMEs, etc. I am sure this District Industrial Profile would be highly beneficial for all the Stakeholders of MSMEs. It is full of academic essence and is expected to provide all kinds of relevant information about the District at a glance. This compilation aims to provide the user a comprehensive insight into the industrial scenario of the district. I would like to appreciate the relentless effort taken by Shri Ravi Kant, Assistant Director (EI) in preparing this informative District Industrial Profile right from the stage of data collection, compilation upto the final presentation. Any suggestion from the stakeholders for value addition in the report is welcome. Place: Patna Date: 31.03.2020 3 Brief Industrial Profile of Bhojpur District 1. General Characteristics of the District– Bhojpur district was carved out of erstwhile Shahbad district in 1992. The Kunwar Singh, the leader of the Mutineers during Sepoy Mutiny in 1857, was from district Bhojpur. -

Lucknow Zone CSC List.Xlsx
Lucknow Zone CSC List Sl. Grampanchayat District Block Name Village/CSC name Pincode Location VLE Name Contact No No. Village Name 1 Sultanpur Sultanpur4 JAISINGHPUR(R) 228125 ISHAQPUR DINESH ISHAQPUR 730906408 2 Sultanpur Baldirai Bhawanighar 227815 Bhawanighar Sarvesh Kumar Yadav 896097886 3 Hardoi HARDOI1 Madhoganj 241301 Madhoganj Bilgram Road Devendra Singh Jujuvamau 912559307 4 Balrampur Balrampur BALRAMPUR(U) 271201 DEVI DAYAL TIRAHA HIMANSHU MISHRA TERHI BAZAR 912594555 5 Sitapur Sitapur Hargaon 261121 Hargaon ashok kumar singh Mumtazpur 919283496 6 Ambedkar Nagar Bhiti Naghara 224141 Naghara Gunjan Pandey Balal Paikauli 979214477 7 Gonda Nawabganj Nawabganj gird 271303 Nawabganj gird Mahmood ahmad 983850691 8 Shravasti Shravasti Jamunaha 271803 MaharooMurtiha Nafees Ahmad MaharooMurtiha 991941625 9 Badaun Budaun2 Kisrua 243601 Village KISRUA Shailendra Singh 5835005612 10 Badaun Gunnor Babrala 243751 Babrala Ajit Singh Yadav Babrala 5836237097 11 Bareilly Bareilly2 Bareilly Npp(U) 243201 TALPURA BAHERI JASVEER GIR Talpura 7037003700 12 Bareilly Bareilly3 Kyara(R) 243001 Kareilly BRIJESH KUMAR Kareilly 7037081113 13 Bareilly Bareilly5 Bareilly Nn 243003 CHIPI TOLA MAHFUZ AHMAD Chipi tola 7037260356 14 Bareilly Bareilly1 Bareilly Nn(U) 243006 DURGA NAGAR VINAY KUMAR GUPTA Nawada jogiyan 7037769541 15 Badaun Budaun1 shahavajpur 243638 shahavajpur Jay Kishan shahavajpur 7037970292 16 Faizabad Faizabad5 Askaranpur 224204 Askaranpur Kanchan ASKARANPUR 7052115061 17 Faizabad Faizabad2 Mosodha(R) 224201 Madhavpur Deepchand Gupta Madhavpur -

Old College New College Colle Attach Ge College Name Course Hon's Subject Colleg Attach College Name
OLD COLLEGE NEW COLLEGE COLLE ATTACH GE COLLEGE NAME COURSE HON'S SUBJECT COLLEG ATTACH COLLEGE NAME 208 PAYHARI MAHARAJ JI COLLEGE, ARA, BHOJPUR B.A. PASS/HON'S 201 HARPRASAD DAS JAIN COLLEGE, BHOJPUR 208 PAYHARI MAHARAJ JI COLLEGE, ARA, BHOJPUR B.COM PASS/HON'S 201 HARPRASAD DAS JAIN COLLEGE, BHOJPUR 208 PAYHARI MAHARAJ JI COLLEGE, ARA, BHOJPUR B.SC PASS/HON'S 201 HARPRASAD DAS JAIN COLLEGE, BHOJPUR 208 PAYHARI MAHARAJ JI COLLEGE, ARA, BHOJPUR B.A. HOME SCIENCE 205 MAHANTH MAHADEVANAND MAHILA MAHAVIDYALYA, ARA 208 PAYHARI MAHARAJ JI COLLEGE, ARA, BHOJPUR B.A. ANCIENT HISTORY 302 SHRI SHANKAR COLLEGE, SASARAM, ROHTAS 208 PAYHARI MAHARAJ JI COLLEGE, ARA, BHOJPUR B.A. SOCIOLOGY 302 SHRI SHANKAR COLLEGE, SASARAM, ROHTAS 219 KUNWER SINGH COLLEGE ,ARA B.A. HOME SCIENCE 205 MAHANTH MAHADEVANAND MAHILA MAHAVIDYALYA, ARA 219 KUNWER SINGH COLLEGE ,ARA B.A. SOCIOLOGY 302 SHRI SHANKAR COLLEGE, SASARAM, ROHTAS 219 KUNWER SINGH COLLEGE ,ARA B.A. PASS/HON'S 202 MAHARAJA COLLEGE , ARA 219 KUNWER SINGH COLLEGE ,ARA B.SC PASS/HON'S 202 MAHARAJA COLLEGE , ARA 219 KUNWER SINGH COLLEGE ,ARA B.COM PASS/HON'S 203 S.B. COLLEGE, ARA 214 S.T.S.M.COLLEGE PANWARI,BHOJPUR B.A. HOME SCIENCE 205 MAHANTH MAHADEVANAND MAHILA MAHAVIDYALYA, ARA 214 S.T.S.M.COLLEGE PANWARI,BHOJPUR B.A. L.S.W. 303 SHERSHAH COLLEGE SASARAM ,ROHTAS 214 S.T.S.M.COLLEGE PANWARI,BHOJPUR B.A. SOCIOLOGY 302 SHRI SHANKAR COLLEGE, SASARAM, ROHTAS 214 S.T.S.M.COLLEGE PANWARI,BHOJPUR B.A. PRAKRIT 203 S.B. -
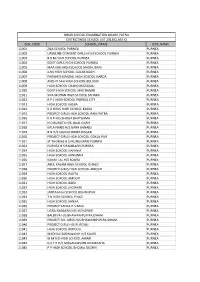
Sch Code School Name Dist Name 11001 Zila School
BIHAR SCHOOL EXAMINATION BOARD PATNA DISTRICTWISE SCHOOL LIST 2013(CLASS X) SCH_CODE SCHOOL_NAME DIST_NAME 11001 ZILA SCHOOL PURNEA PURNEA 11002 URSULINE CONVENT GIRLS HIGH SCHOOL PURNEA PURNEA 11003 B B M HIGH SCHOOL PURNEA PURNEA 11004 GOVT GIRLS HIGH SCHOOL PURNEA PURNEA 11005 MAA KALI HIGH SCHOOL MADHUBANI PURNEA 11006 JLNS HIGH SCHOOL GULAB BAGH PURNEA 11007 PARWATI MANDAL HIGH SCHOOL HARDA PURNEA 11008 ANCHIT SAH HIGH SCHOOL BELOURI PURNEA 11009 HIGH SCHOOL CHANDI RAZIGANJ PURNEA 11010 GOVT HIGH SCHOOL SHRI NAGAR PURNEA 11011 SIYA MOHAN HIGH SCHOOL SAHARA PURNEA 11012 R P C HIGH SCHOOL PURNEA CITY PURNEA 11013 HIGH SCHOOL KASBA PURNEA 11014 K D GIRLS HIGH SCHOOL KASBA PURNEA 11015 PROJECT GIRLS HIGH SCHOOL RANI PATRA PURNEA 11016 K G P H/S BHOGA BHATGAMA PURNEA 11017 N D RUNGTA H/S JALAL GARH PURNEA 11018 KALA NAND H/S GARH BANAILI PURNEA 11019 B N H/S JAGNICHAMPA NAGAR PURNEA 11020 PROJECT GIRLS HIGH SCHOOL GOKUL PUR PURNEA 11021 ST THOMAS H S MUNSHIBARI PURNEA PURNEA 11023 PURNEA H S RAMBAGH,PURNEA PURNEA 11024 HIGH SCHOOL HAFANIA PURNEA 11025 HIGH SCHOOL KANHARIA PURNEA 11026 KANAK LAL H/S SOURA PURNEA 11027 ABUL KALAM HIGH SCHOOL ICHALO PURNEA 11028 PROJECT GIRLS HIGH SCHOOL AMOUR PURNEA 11029 HIGH SCHOOL RAUTA PURNEA 11030 HIGH SCHOOL AMOUR PURNEA 11031 HIGH SCHOOL BAISI PURNEA 11032 HIGH SCHOOL JHOWARI PURNEA 11033 JANTA HIGH SCHOOL BISHNUPUR PURNEA 11034 T N HIGH SCHOOL PIYAZI PURNEA 11035 HIGH SCHOOL KANJIA PURNEA 11036 PROJECT KANYA H S BAISI PURNEA 11037 UGRA NARAYAN H/S VIDYAPURI PURNEA 11038 BALDEVA H/S BHAWANIPUR RAJDHAM -

Gorakhpur- Faizabad of Electoral Rolls of Teachers'constituency 2019
Gorakhpur- Faizabad of Electoral Rolls of Teachers'Constituency 2019 Constituency Header - Gorakhpur- Faizabad Teachers'Constituency Part Header- Block Deblopment Office- pachperwa Part No. of Electoral Roll- 185 Part Summary- Block Development Pachperwa, Town Area Pachperwa Constituency Summary- Gorakhpur- Faizabad Teachers'Constituency dist- Balrampur Name Name of No. of of Part State N0.of Part of of where Assem Assembl Assemb Name(s) of specified elector is bly y ly Serial educational insitution(s) enrolled Constit Name of Constitu Constitu Number in where engaged in in uency Assembly ency ency Part where Relation teaching for three years Assembl where Constituency where where elector is Surname Type(Fathe out of last six years SL No. Surname of Epic y elector where elector elector is elector enrolled(if First Name of Elector Name of Relation of r/Mother/H Address Sex Date of Birth (names of all such Photo in Part Elector Number Consitue is is enrolled (if enrolled is enrolled in Relation usband/Oth educational insitutions ncy (if enrolled enrolled in (if enrolled any er should be given in which enrolled in any any assembly enrolled (if assembly the elector has been in any assemb constituency) in any enrolled constituency engaged in teaching in the assembl ly assembl in any ) last six years) y constitu y assemb constitue ency) constiyu ly ncy) ency) constitu ency) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 UP 292 GAINSARI && VILL. JUDIKUINYA POST. MADARSA GARIB NAWAZ 1 ADIL AHMAD SRI TUFAIL AHMAD FATHER PACHPERWA M 1/1/1991 BARGADWA SAIF Photo BALRAMPUR BALRAMPUR & UP 292 GAINSARI && VILL. -

Nakaha Jungle - Lucknow Jn
Sep 30 2021 (00:39) India Rail Info 1 Nakaha Jungle - Lucknow Jn. Passenger (UnReserved)/55031 - Pass - NER TLR/Tulsipur to LJN/Lucknow Junction NER 8h 58m - 195 km - 31 halts - Departs Daily # Code Station Name Arrives Avg Depart Avg Halt PF Day Km Spd Elv Zone s 1 JEA Nakaha Jungle 07:45 2 1 0 29 84 NER 2 MIM Maniram 07:55 07:57 2m 1 1 5 49 84 NER 3 JKI Kauriaa Jungle 08:04 08:05 1m 0 1 11 35 86 NER 4 PJ Peppeganj 08:15 08:17 2m 0 1 16 27 85 NER 5 RJ Rawat Ganj 08:24 08:25 1m 0 1 20 36 88 NER 6 MVKR Mahawan Khor Halt 08:29 08:30 1m 0 1 22 36 84 NER 7 RCRA Ramchaura 08:34 08:35 1m 0 1 24 50 83 NER 8 CM Campierganj 08:41 08:43 2m 0 1 29 23 84 NER 9 LPW Loharpurwa 08:52 08:53 1m 0 1 33 16 85 NER 10 ANDN Anand Nagar Junction 09:05 09:10 5m 3 1 36 77 NER 11 LER Lehra 09:17 09:18 1m 0 1 45 67 NER 12 BMJ Bridgemanganj 09:22 09:24 2m 0 1 49 62 NER 13 UB Uska Bazar 09:32 09:34 2m 0 1 58 36 NER 14 NUH Naugarh 09:50 09:55 5m 1 1 67 70 NER 15 AHU Ahirauli 10:00 10:01 1m 0 1 73 32 NER 16 CIH Chilhia 10:13 10:15 2m 0 1 80 31 NER 17 SOT Shohratgarh 10:27 10:29 2m 0 1 86 56 NER 18 MHBZ Mahtha Bazar 10:34 10:35 1m 0 1 91 14 NER 19 PRZ Parsa 10:55 10:57 2m 0 1 95 38 NER 20 MHBG Mahadev Buzurg 11:07 11:08 1m 0 1 101 16 NER 21 BNY Barhni 11:30 11:35 5m 1 1 107 70 NER 22 TPB Trilokpur 11:42 11:43 1m 0 1 116 26 NER 23 PPW Pachperwa 12:00 12:02 2m 0 1 123 112 NER 24 GIR Gainsari Junction 12:07 12:09 2m 0 1 132 51 NER 25 LBW Laiburwa Halt 12:18 12:19 1m 0 1 140 26 116 NER 26 TLR Tulsipur 12:32 12:37 5m 0 1 146 61 112 NER 27 LHNR Lakshmanpur Halt 12:44 12:45 -

DOS Name of GSS: ARA LIST of EXISTING EQUIPMENT DETAILS
Name of Circle: DOS Name of GSS: ARA LIST OF EXISTING EQUIPMENT DETAILS Sl. Date of Name of Bay Equipment Name Quantity Make No. Commissioning Line control & relay panel with Distance 01 ALSTOM 2015 protection & O/C E/F protection 3 Phase Circuit Breaker 01 ALTOM 2015 1-Ph CVT 03 2017 PGCIL (ARA) LA 03 2016 1-Ph Current Transformer 03 SCT LIMITED 3 Phase Isolator 132 KV Bay with/without Earth switch 03 FARADAY 2015 Line control & relay panel with Distance 01 ALSTOM 2017 protection & O/C E/F Railway protection feeder 2 Phase Circuit Breaker 01 CGL 2017 LA 03 2015 Line control & relay panel with Distance protection & O/C E/F ALSTOM protection 01 2015 3 Phase Circuit Breaker 01 ALSTOM 2015 132 KV B/C bay B/C MEHRU 1 Ph PT 03 VOLTAGE 2015 LA 2015 1-Ph Current Transformer 03 Artche 3 Phase Isolator with/without Earth switch 03 FARADAY 2015 Transformer control & T-1to3 – relay panel with ALSTOM Differential protection T-4-VENSON & O/C E/F protection 04 ELECT. 2015 T-1-CGL T-2- ALSTOM 3 Phase Circuit Breaker T-3-CGL 04 T-4-CGL LA 12 2016 T-1&2- 132/33 KV Transformer VISHAL PVT bay(HV) LTD 1-Ph Current T-3-SCT LTD Transformer T-4- HEPTACARE Transformer POWER 12 INDUSRT T-1&2- 3 Phase Isolator FARADAY T 1&2-2016 with/without Earth T-3&4-- T3-2014 switch 12 Simens T4-2016 Transformer control & T-1 TO 3- 132/33 KV Transformer relay panel with ALSTOM bay(LV) Differential protection T-4-VENSON Transformer & O/C E/F protection 04 ELECT.