How to Improve the Antioxidant Defense in Asphyxiated Newborns—Lessons from Animal Models
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Motion Sickness: Definition
Clinical and physiological characteristics of cybersickness Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy (Human Physiology) School of Biomedical Sciences and Pharmacy Faculty of Health and Medicine University of Newcastle, Australia This research was supported by an Australian Government Research Training Program (RTP) Scholarship. 1 STATEMENT OF ORIGINALITY I hereby certify that the work embodied in the thesis is my own work, conducted under normal supervision. The thesis contains no material which has been accepted, or is being examined, for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made. I give consent to the final version of my thesis being made available worldwide when deposited in the University’s Digital Repository, subject to the provisions of the Copyright Act 1968 and any approved embargo. Alireza Mazloumi Gavgani 2 ACKNOWLEDGMENT OF AUTHORSHIP I hereby certify that the work embodied in this thesis contains published paper/s/scholarly work of which I am a joint author. I have included as part of the thesis a written declaration endorsed in writing by my supervisor, attesting to my contribution to the joint publication/s/scholarly work. Alireza Mazloumi Gavgani 3 STATEMENT OF COLLABORATION I hereby certify that some parts of the work embodied in this thesis have been done in collaboration with other researchers. I have included as part of the thesis a statement clearly outlining the extent of collaboration, with whom and under what auspices. -

Hyperthermia & Heat Stroke: Heat-Related Conditions
Hyperthermia & Heat Stroke: Heat-Related Conditions Joseph Rampulla, MS, APRN,BC eat-related conditions occur when excess heat taxes or overwhelms the body’s thermoregulatory mechanisms. Heat illness is preventable and occurs more Hcommonly than most clinicians realize. Heat illness most seriously affects the poor, urban-dwellers, young children, those with chronic physical and mental illnesses, substance abusers, the elderly, and people who engage in excessive physical The exposure to activity under harsh conditions. While considerable overlap occurs, the important the heat and the concrete during the syndromes are: heat stroke, heat exhaustion, and heat cramps. Heat stroke is a life- hot summer months places many rough threatening emergency and occurs when the loss of thermoregulatory control results sleepers at great risk in hyperpyrexia (very high fever) and severe damage to many internal organs. for heat stroke and hyperthermia. Photo by Epidemiology Sharon Morrison RN Heat illness is generally underreported, and the deaths than all other natural disasters combined in true incidence is unknown. Death rates from other the USA. The elderly, the very poor, and socially causes (e.g. cardiovascular, respiratory) increase isolated individuals are disproportionately affected during heat waves but are generally not reflected in by heat waves. For example, death records during the morbidity and mortality statistics related to heat heat waves invariably include many elders who died illness. Nonetheless, heat waves account for more alone in hot apartments. Age 65 years, chronic The Health Care of Homeless Persons - Part II - Hyperthermia and Heat Stroke 199 illness, and residence in a poor neighborhood are greater than 65. -

Hypothermia Hyperthermia Normothemic
Means normal body temperature. Normal body core temperature ranges from 99.7ºF to 99.5ºF. A fever is a Normothemic body temperature of 99.5 to 100.9ºF and above. Humans are warm-blooded mammals who maintain a constant body temperature (euthermia). Temperature regulation is controlled by the hypothalamus in the base of the brain. The hypothalamus functions as a thermostat for the body. Temperature receptors (thermoreceptors) are located in the skin, certain mucous membranes, and in the deeper tissues of the body. When an increase in body temperature is detected, the hypothalamus shuts off body mechanisms that generate heat (for example, shivering). When a decrease in body temperature is detected, the hypothalamus shuts off body mechanisms designed to cool the body (for example, sweating). The body continuously adjusts the metabolic rate in order to maintain a constant CORE Hypothermia Core body temperatures of 95ºF and lower is considered hypothermic can cause the heart and nervous system to begin to malfunction and can, in many instances, lead to severe heart, respiratory and other problems that can result in organ damage and death.Hannibal lost nearly half of his troops while crossing the Pyrenees Alps in 218 B.C. from hypothermia; and only 4,000 of Napoleon Bonaparte’s 100,000 men survived the march back from Russia in the winter of 1812 - most dying of starvation and hypothermia. During the sinking of the Titanic most people who entered the 28°F water died within 15–30 minutes. Symptoms: First Aid : Mild hypothermia: As the body temperature drops below 97°F there is Call 911 or emergency medical assistance. -

Malignant Hyperthermia.Pdf
Malignant Hyperthermia Matthew Alcusky PharmD, MS Student University of Rhode Island July, 2013 Financial Disclosure I have no financial obligations to disclose. Outline • Introduce malignant hyperthermia including its causes and implications • Describe the underlying pathophysiology • Detail the clinical presentation of MH • Summarize the necessary pharmacological and non-pharmacological treatment of MH • Highlight necessary considerations with the use of dantrolene • Discuss recrudescence Malignant Hyperthermia • A life threatening reaction that is most often triggered by the use of inhalational anesthetics • Estimated incidence of 1 in 5,000 to 1 in 100,000 anesthesia inductions • Early recognition and treatment is essential in reducing morbidity and mortality • Screening patients for past anesthesia history and family history, as well as conducting testing on at risk individuals is necessary to reduce MH occurrence Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007 Apr 24;2:21. Review. Drugs Triggering Malignant Hyperthermia • Desflurane • Succinylcholine- • Enflurane only non-inhalational • Halothane anesthetic that triggers MH • Isoflurane • Nitrous Oxide- only • Methoxyflurane inhalational anesthetic • Sevoflurane that does not cause MH Hopkins PM. Malignant hyperthermia: pharmacology of triggering. Br J Anaesth. 2011 Jul;107(1):48-56. doi: 10.1093/bja/aer132. Epub 2011 May 30. Review. Pathophysiology • MH partially attributed to a dominant mutation in the ryanodine receptror 1 (RYR1) – Ryanodine receptors are activated by elevated Ca2+ levels, known as store overload induced calcium release (SOICR) – Mutant receptors are activated by lower Ca2+ levels – Volatile anesthetics further lower the SOICR threshold MacLennan DH, Chen SR. Store overload-induced Ca2mutations + release as a triggering mechanism for CPVT and MH episodes caused by in RYR and CASQ genes. -

Guidelines for Management of a Malignant Hyperthermia (MH) Crisis
Guidelines for the management of a Malignant Hyperthermia Crisis Successful treatment of a Malignant Hyperthermia (MH) crisis depends on early diagnosis and aggressive treatment. The onset of a reaction can be within minutes of induction or may be more insidious. Previous uneventful anaesthesia does not exclude MH. The steps below are intended as an aide memoire. Presentation may vary and treatment should be modified accordingly. Know where the dantrolene is stored in your theatre. Treatment can be optimised by teamwork. Call for help Diagnosis - consider MH if: 1 1. Unexplained, unexpected increase in end-tidal CO2 together with 2. Unexplained, unexpected tachycardia together with 3. Unexplained, unexpected increased in oxygen consumption Masseter muscle spasm, and especially more generalised muscle rigidity after suxamethonium, indicate a high risk of MH susceptibility but are usually self-limiting. Take measures to halt the MH process: 2 1. Remove trigger drugs, turn off vaporisers, use high fresh gas flows (oxygen), use a new, clean non-rebreathing circuit, hyperventilate. Maintain anaesthesia with intravenous agents such as propofol until surgery completed. 2. Dantrolene; give 2-3 mg.kg-1 i.v. initially and then 1 mg.kg-1 PRN. 3. Use active body cooling but avoid vasoconstriction. Convert active warming devices to active cooling, give cold 3 intravenous infusions, cold peritoneal lavage, extracorporeal heat exchange. Monitor: 4 ECG, SpO2, end-tidal CO2, invasive arterial BP, CVP, core and peripheral temperature, urine output and pH, arterial blood gases, potassium, haematocrit, platelets, clotting indices, creatine kinase (peaks at 12-24h). Treat the effects of MH: 5 1. Hypoxaemia and acidosis: 100% O2, hyperventilate, sodium bicarbonate. -
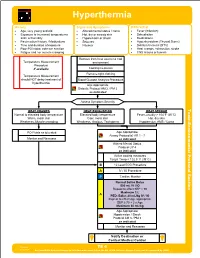
TE 04 Hyperthermia Protocol Final 2017 Editable.Pdf
Hyperthermia History Signs and Symptoms Differential • Age, very young and old • Altered mental status / coma • Fever (Infection) • Exposure to increased temperatures • Hot, dry or sweaty skin • Dehydration and / or humidity • Hypotension or shock • Medications • Past medical history / Medications • Seizures • Hyperthyroidism (Thyroid Storm) • Time and duration of exposure • Nausea • Delirium tremens (DT's) • Poor PO intake, extreme exertion • Heat cramps, exhaustion, stroke • Fatigue and / or muscle cramping • CNS lesions or tumors Remove from heat source to cool Temperature Measurement environment Procedure if available Cooling measures Remove tight clothing Temperature Measurement should NOT delay treatment of Blood Glucose Analysis Procedure hyperthermia Age Appropriate Diabetic Protocol AM 2 / PM 2 as indicated Assess Symptom Severity Toxic HEAT CRAMPS HEAT EXHAUSTION HEAT STROKE Normal to elevated body temperature Elevated body temperature Fever, usually > 104°F (40°C) Warm, moist skin Cool, moist skin Hot, dry skin - Weakness, Muscle cramping Weakness, Anxious, Tachypnea Hypotension, AMS / Coma Section Environmental Protocol PO Fluids as tolerated Age Appropriate Airway Protocol(s) AR 1 - 7 Monitor and Reassess as indicated Altered Mental Status Protocol UP 4 as indicated Active cooling measures Target Temp < 102.5° F (39°C) B 12 Lead ECG Procedure A IV / IO Procedure P Cardiac Monitor A Age Appropriate Hypotension / Shock Protocol AM 5 / PM 3 as indicated Monitor and Reassess Notify Destination or Contact Medical Control Revised TE 4 09/29/2017 Any local EMS System changes to this document must follow the NC OEMS Protocol Change Policy and be approved by OEMS Hyperthermia Toxic - Environmental Protocol Environmental Section Protocol Pearls • Recommended Exam: Mental Status, Skin, HEENT, Heart, Lungs, Neuro • Extremes of age are more prone to heat emergencies (i.e. -

An Updated Narrative Review on Ergometric Systems Applied to Date in Assessing Divers’ Fitness
healthcare Review An Updated Narrative Review on Ergometric Systems Applied to Date in Assessing Divers’ Fitness Sven Dreyer 1, Johannes Schneppendahl 1, Fabian Moeller 2, Andreas Koch 3, Thomas Muth 4 and Jochen D Schipke 5,* 1 Hyperbaric Oxygen Therapy, University Hospital Düsseldorf, Moorenstrasse 5, 40225 Düsseldorf, Germany; [email protected] (S.D.); [email protected] (J.S.) 2 Department of Exercise Physiology, Institute of Exercise Training and Sport Informatics, German Sport University Cologne, 50933 Cologne, Germany; [email protected] 3 German Naval Medical Institute, Maritime Medicine, 24119 Kronshagen, Germany; [email protected] 4 Institute of Occupational, Social and Environmental Medicine, Medical Faculty and University Hospital Düsseldorf, Heinrich-Heine-University, 40225 Düsseldorf, Germany; [email protected] 5 Forschungsgruppe Experimentelle Chirurgie, Universitäts-Klinikum Düsseldorf, Moorenstrasse 5, 40225 Düsseldorf, Germany * Correspondence: [email protected]; Tel.: +49-211-57-99-94 Abstract: Many recreational divers suffer medical conditions, potentially jeopardizing their safety. To scale down risks, medical examinations are mandatory and overwhelmingly performed using bicycle ergometry, which overlooks some important aspects of diving. Searching ergometric systems that better address the underwater environment, a systematic literature search was conducted using the keywords ‘diving’, ‘fitness’, ‘ergometry’, and ‘exertion’. All presented alternative systems Citation: Dreyer, S.; Schneppendahl, found convincingly describe a greatly reduced underwater physical performance. Thus, if a diver’s J.; Moeller, F.; Koch, A.; Muth, T.; workload in air should already be limited, he/she will suffer early from fatigue, risking a diving Schipke, J.D. An Updated Narrative incident. How to assess fitness? Performance diagnostics in sports is always specific for a modality Review on Ergometric Systems or movement. -

Care of the Addicted Patient
CARE OF THE ADDICTED PATIENT Andrew Linnaus, MD DISCLOSURE • No financial relationships or conflicts of interest to disclose. GOALS • Review Traditional Toxidromes and associated drugs of abuse • Review Emerging Drugs of Abuse • Discuss the Current Opiate Epidemic • Discuss care for the recovering/addicted patient. SYMPATHOMIMETIC SYMPATHOMIMETIC • Cocaine, Amphetamines, LSD, Ectasy (MDMA) SYMPATHETIC RESPONSE • Eyes (Alpha 1): Pupil Dilation/Mydriasis • Heart (Beta 1): Increased HR, Contraction, irritability • Often sinus tachycardia but at risk for arrythmias. • Lungs (Beta2): Dilation of bronchial tree • Skin (Alpha 1): Sweating, goose bumps • Muscles (Beta2): Vasodilation-increased blood flow. • Tachycardia, Hypertension, Hyperthermia SYMPATHETIC RESPONSE • Physical Exam • Tremor, warm skin, diaphoresis, hypoactive bowel sounds. • Sympathetic stimulation Epinephrine (adrenalin) release form the adrenal glands • “Fight or flight” SYMPATHOMIMETIC-HYPERTHERMIA • Hyperthermia • Increased motor tone generates heat • Can be exacerbated by dehydration and vasoconstriction • Temp >106 F documented DIC and multisystem organ failure • Treatment • Aggressive use of fluid and benzodiazepines. • Neuromuscular paralysis and intubation as needed. • Continuous monitoring of core temperature-rectal probe. • Wet sheets with large fans, pack groin and axilla with ice packs • Goal of 102 or less within 20 minutes SYMPATHOMIMETIC-HYPERTENSION • Hypertensive Emergencies • Aortic Dissection, Pulmonary Edema, Myocardial Ischemia/Infarction, Intracranial Hemorrhage, -
Key Words for Diving and Hyperbaric Medicine
2 Index to Diving and Hyperbaric Medicine for 2009, Volume 39 KEY WORDS FOR DIVING AND HYPERBARIC MEDICINE Abalone Data Gasinduced osmosis Abstracts Deaths Gas solubility Accidents Decompression General interest Adolescents Decompression illness Genitourinary tract Aerobic capacity Decompression sickness Gleanings (from medical journals) Age Deep diving Haematology Air DES – Diver Emergency Service Health Air embolism Diabetes Health status Allergy DIMS Health surveillance Altitude Disabilities Health surveys Anaesthesia Disability Hearing Antarctica Disabled diver Helium pharmacokinetics Arterial gas embolism Diver numbers Hip arthroplasty Ascent Diving History Asthma Diving accidents Human skin equivalent Autobiography Diving at work Hyperbaric facilities Autopsy Diving deaths Hyperbaric oxygen Aviation Diving industry Hyperbaric oxygen therapy Barotrauma Diving organisations Hyperbaric oxygenation Bell diving Diving reflex Hyperbaric research Beta blockade Diving research Hypercapnia Biology Diving safety memos Hyperthermia (see Thermal problems) Blood pressure Diving scholars Hyperventilation Blood substitutes Diving tables Hypothermia (see Thermal problems) Blood sugar level Diving theory (see Physiology) Hypoxia Book reviews Doppler Ice Brain injury Drowning Immersion Breathhold diving Drugs Immunosuppression Bronchial provocation testing Drysuit Incidents Bubbles Dysbaric osteonecrosis Infectious diseases Buccal pumping Ear barotrauma Inflammation Buddies Ear infection Injuries Buoyancy Echocardiography Inner ear Calciphylaxis Ecology -

Hypothermia Therapy for Newborns with Hypoxic Ischemic Encephalopathy
J Pediatr (Rio J). 2015;91(6 Suppl 1):S78---S83 www.jped.com.br ARTIGO DE REVISÃO Hypothermia therapy for newborns with hypoxic ଝ ischemic encephalopathy a,b a,b,∗ Rita C. Silveira e Renato S. Procianoy a Departamento de Pediatria, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brasil b Servic¸o de Neonatologia, Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brasil Recebido em 31 de julho de 2015; aceito em 3 de agosto de 2015 KEYWORDS Abstract Hypothermia; Objective: Therapeutic hypothermia reduces cerebral injury and improves the neurological Neonates; outcome secondary to hypoxic ischemic encephalopathy in newborns. It has been indicated for asphyxiated full-term or near-term newborn infants with clinical signs of hypoxic-ischemic Perinatal asphyxia; Hypoxic-ischemic encephalopathy (HIE). encephalopathy; Sources: A search was performed for articles on therapeutic hypothermia in newborns with Outcomes perinatal asphyxia in PubMed; the authors chose those considered most significant. Summary of the findings: There are two therapeutic hypothermia methods: selective head coo- ◦ ling and total body cooling. The target body temperature is 34.5 C for selective head cooling and ◦ ◦ 33.5 C for total body cooling. Temperatures lower than 32 C are less neuroprotective, and tem- ◦ peratures below 30 C are very dangerous, with severe complications. Therapeutic hypothermia must start within the first 6 hours after birth, as studies have shown that this represents the therapeutic window for the hypoxic-ischemic event. Therapy must be maintained for 72 hours, with very strict control of the newborn’s body temperature. It has been shown that therapeutic hypothermia is effective in reducing neurologic impairment, especially in full-term or near-term newborns with moderate HIE. -

Diving and Hyperbaric Medicine the Journal of the South Pacific Underwater Medicine Society (Incorporated in Ictoria)V A0020660B
Diving and Hyperbaric Medicine The Journal of the South Pacific Underwater Medicine Society (Incorporated in ictoria)V A0020660B ISSN 1833 3516 Volume 37 No. 3 ABN 29 299 823 713 September 2007 Aerobic fitness and scuba diving Deep decompression stops – do they improve safety? HBO and cancer – friend or foe? The man who thought his wife was a hat Improving diving medicine courses Print Post Approved PP 331758/0015 CONTENTS Diving and Hyperbaric Medicine Volume 37 No. 3 September 2007 Editorial SPUMS notices & news 117 The Editor’s offering – diving medicine education 152 Diploma of Diving and Hyperbaric Medicine requirements 152 Approved extracts of minutes of the SPUMS Executive Review articles Committee Meeting, held on 118 Aerobic fitness and underwater diving 19 April 2007 at Oceans Resort, Neal W Pollock Tutukaka, New Zealand 125 Deep decompression stops 153 Dates and venues of the SPUMS Andrew Fock Annual Scientific Meetings 154 Greetings from the new SPUMS Webmaster 155 ANZ College of Anaesthetists SPUMS ASM 2007 Special Interest Group in Diving 133 Hyperbaric oxygenation in the patient with malignancy: and Hyperbaric Medicine (SIG- friend or foe? DHM) Heather M Macdonald Case report Letter to the Editor 139 Transient prosopagnosia resulting from a cerebral gas 164 Project Stickybeak and DAN embolism while diving AP dive accident reporting Colin M Wilson, Martin DJ Sayer and A Gordon Murchison project John Lippmann Short communication 143 Effects of a single hyperbaric oxygen exposure on haematocrit, prothrombin time, serum calcium, -

Hyperthermia to Treat Cancer
cancer.org | 1.800.227.2345 Hyperthermia to Treat Cancer Hyperthermia usually is taken to mean a body temperature that is higher than normal. High body temperatures are often caused by illnesses, such as fever or heat stroke. But hyperthermia can also refer to heat treatment – the carefully controlled use of heat for medical purposes. Here, we will focus on how heat is used to treat cancer. When cells in the body are exposed to higher than normal temperatures, changes take place inside the cells. These changes can make the cells more likely to be affected by other treatments such as radiation therapy1 or chemotherapy2. Very high temperatures can kill cancer cells outright (thermal ablation), but they also can injure or kill normal cells and tissues. This is why hyperthermia must be carefully controlled and should be done by doctors who are experienced in using it. Current instruments can deliver heat precisely, and hyperthermia is being used (or studied for use) against many types of cancer. How is hyperthermia used to treat cancer? Treatment can be local, regional or whole-body hyperthermia, depending on the extent of the area being treated. Local hyperthermia Local hyperthermia is used to heat a small area like a tumor. Very high temperatures are used to kill the cancer cells and destroy nearby blood vessels. In effect, this cooks the area that is exposed to the heat. And, as with cooking, the higher the temperature and duration of exposure, the greater the effect seen within tissues. Thermal ablation comprises the treatments where very high temperatures cause irreversible damage to cells whereas smaller rises in temperature constitute mild hyperthermia.