Downloaded from Course at a University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Goessmann, Lindsey, Chamberlain, Peters, and Mcewen, Research Symposium
GOE SSMANNgazette A Publication of the Chemistry Department University of Massachusetts Amherst www.chem.umass.edu VOLUME 44 – SPRING 2015 INSIDE Alumni News ............................2 by David Adams Points of Pride ...........................4 Chemistry Loses a Dear Friend Lab Notes .................................5 Dissertation Seminars .............21 On April 14th one of the towering figures of the Chemistry Seminar Program ....................20 Department, Professor George R. Richason, Jr. passed away Senior Awards Dinner .............22 at Cooley Dickinson Hospital in Northampton. Alongside Degrees Awarded ...................22 Goessmann, Lindsey, Chamberlain, Peters, and McEwen, Research Symposium ..............23 George takes his place among the chemists who shaped Friends of Chemistry ...............26 and propelled the department to national and international Letter from Head ....................28 quality and recognition. In George’s case, he was part of EVENTS for 2015 the Chemistry Department for 82 of its 146 year history! His contributions to the department and the university Five College Seminar were profound, widespread, and legendary. In many Prof. Phil Baran Scripps Institute respects he truly was “Mr. UMass.” March 10, 2015 In the early 1930s, George, born in the Riverside Marvin Rausch Lectureship Prof. Karl Wieghardt section of Turner’s Falls on April 3, 1916, participated in Max-Planck-Institut-Mülheim basketball tournaments on the Amherst campus of the then April 9, 2015 Massachusetts Agricultural College (MAC). MAC became Senior Awards Dinner Massachusetts State College in 1931, and George April 29, 2015 matriculated at MSC in the fall of 1933. Early in his undergraduate career the basketball coach Getting to Know Our Newest Alumni Reunion 2015 June 6, 2015 encouraged him to join the State basketball team Faculty Members after watching him play in Curry Hicks Cage. -
A Day of Rage in Greece As Debt Worries Mount
O C V ΓΡΑΦΕΙ ΤΗΝ ΙΣΤΟΡΙΑ Bringing the news ΤΟΥ ΕΛΛΗΝΙΣΜΟΥ to generations of ΑΠΟ ΤΟ 1915 The National Herald Greek Americans c v A wEEkly GREEk AMERICAN PuBlICATION www.thenationalherald.com VOL. 14, ISSUE 698 February 26-March 4 , 2011 $1.50 A Day of Rage in Greece as Debt Worries Mount Back to the Drach? Some Analysts Say Restructuring Inevitable, Is Coming ATHENS – Pumped up by up - loans over three years to keep risings in other countries, more the country from going bank - than 30,000 protesters furious rupt. Prime Minister George Pa - over government-imposed pay pandreou has acknowledged cuts for public workers, tax that generations of profligate hikes and an international cadre overspending by different gov - of lenders who have nearly ernment administrations has taken control of the country’s fi - created the crisis he said left nances, clashed with riot police him no chance but to seek in - on Feb. 23 during a day-long ternational help, but at a price general strike that shut down many citizens said is too heavy businesses, services and trans - and has exempted the rich and portation. Graffiti calling for a politicians they blame for the “Day of Rage,” the calling cry of dilemma. Protesters chanting demonstrators who overthrew “Don’t obey the rich — Fight the Egyptian government and back!” marched to Parliament set off a spate of uprisings in as the city center was heavily Yemen and Libya and unease in policed. northern Africa and the ISLANDS NOT FOR SALE Mideast, was sprawled on walls The assault on Parliament, in the capital -

Library Director and University Librarian
LIBRARY DIRECTOR AND UNIVERSITY LIBRARIAN The University of Chicago invites applications and nominations for the position of Library Director and University Librarian. Reporting to the Provost, the Library Director is a critical partner in, and facilitator of, the rigorous intellectual engagement that characterizes the University of Chicago. The new Library Director will have a tremendous opportunity to work across the entire university, building upon the Library’s exceptional service-oriented culture in order to further the rigorous academic goals of the University. Working with a team of approximately 200 talented and dedicated library staff, the new Library Director will lead the process of developing and implementing a comprehensive strategic vision for the future of the Library, in terms of both its role within the University ecosystem and its relationship to the fast-changing world of information management. The new Library Director will be an experienced and effective leader who will be a champion for the centrality of the library in an academic research university. The new Library Director should bring a creative, intellectual, and collaborative spirit to the challenge of making a library that is already well respected and beloved for the depth of its collections and prominence both on- and off-campus even more central to the University of Chicago’s mission and even more recognized around the nation and world. The Joseph Regenstein Library University of Chicago, Library Director and University Librarian Page 2 ABOUT THE UNIVERSITY The University of Chicago is a research university in a dynamic urban setting that has driven new ways of thinking since 1890. -

A. Paul Alivisatos to Receive 2011 Von Hippel Award for Colloidal
SOCIETY NEWS of these quantum dots may enable new types of solar photovoltaic and solar A. Paul Alivisatos fuel generators. In the last decade, Alivisatos has to receive 2011 demonstrated the emergence of “arti¿ cial molecules,” where small numbers of col- Von Hippel Award loidal nanoparticles are joined together into speci¿ c “molecular” arrangements for colloidal nanoparticles with controlled symmetry and connectiv- ity. These new “molecular” systems have opened a new area of materials for discovery and innovation. An important breakthrough by Alivisatos he 2011 Von Hippel Award, the and growth, as well as the time scales in this area has been the demonstration TMaterial Research Society’s high- involved, and upon the concept of “size of the “plasmon ruler,” which, for ex- est honor, will be presented to A. Paul distribution focusing,” in which the ample, can measure the distance between Alivisatos, director of the Lawrence distribution of nanoparticle sizes is nar- two Au nanocrystals joined by DNA or Berkeley National Laboratory and Larry rowed when small particles grow faster peptides to measure dynamical distance and Diane Bock Professor of Nanotech- than large ones. Proof of the high quality changes in biological systems. nology, University of California–Berke- of nanocrystals is gleaned from studies Another important area Alivisatos ley. Alivisatos is being recognized for of optical properties, as well as from has pioneered is the study of chemical “the development of the fundamental studies of the structural transformations transformations of colloidal nanocrys- scienti¿ c basis for growing and utiliz- of nanoparticles under high pressure, tals. He has also demonstrated methods ing defect-free colloidal semiconductor demonstrating the absence of defects. -

ACS Division of Inorganic Chemistry
American Chemical Society Division of Inorganic Chemistry DIC Web- Site: http://membership.acs.org/i/ichem/index.html Department of Chemistry, Texas A&M University, PO Box 30012, College Station, TX 77842-3012 979 845-5235, [email protected] 2004 Officers Prepared by Kim R. Dunbar, Secretary Al Sattelberger Chair Clifford P. Kubiak 1. ELECTION 2004 Chair-Elect Following is the list of offices to be filled and the candidates for each: Kim Dunbar Secretary • Chair-Elect: Peter C. Ford and Thomas B. Rauchfuss William E. Buhro • Treasurer-Elect: Donald H. Berry and Mary P. Neu Secretary-Elect Bryan Eichhorn • Executive Committee Member at Large: Treasurer Kristen Bowman-James and George G. Stanley Subdivision Chairs • Councilors (2 will be elected: William B. Tolman Jeffrey R. Long, Philip P. Power, Gregory H. Robinson and Lawrence R. Sita Bioinorganic • Alternate Councilors (2 will be elected): Janet Morrow Bioinorganic-Elect Sonya J. Franklin, François P. Gabbaï, Jonas C. Peters and John D. Protasiewicz Patricia A. Shapley • Chair-Elect, Bioinorganic: A.S. Borovik and Joan B. Broderick Organometallic • Chair-Elect, Organometallic: R. Morris Bullock and Gerard Parkin Klaus H. Theopold Organometallic-Elect • Chair-Elect, Solid State and Materials Chemistry: Hanno zur Loye David C. Johnson and Omar M. Yaghi Solid State • Chair-Elect, Nanoscience: Thomas E. Mallouk and Chad A. Mirkin Edward G. Gillan Solid State-Elect Peidong Yang 2. MESSAGE FROM THE CHAIR – Al Sattelberger Nanoscience This summer's International Conference on Coordination Chemistry (ICCC-36) was James E. Hutchison Nanoscience-Elect a major accomplishment for the DIC. Over 1100 participants representing inorganic chemistry programs from around the world attended the conference in Merida, Executive Committee Mexico on July 18-23. -

William E. Mahoney Annual Lecture in Chemistry
ILLIAM E. MAHONEY is a 1955 alumnus of OBERT M. MAHONEY W the Department of Chemistry at i R is President and Chief the University of Massachusetts, Executive Officer of Belmont Amherst. Professor Mahoney was Savings Bank. Vice Chairman and Chief Operating Officer, as well as Chairman of the Mahoney received his M.B.A. Executive Committee of the Board of from Columbia Business School Directors, of Witco Corporation (now in 1971. He is a 1970 graduate of Chemtura Corporation), a Fortune 500 the University of Massachusetts, manufacturer of specialty chemical and petroleum products. where he earned a Bachelor of Science degree in Chemistry. He received the 1996 After retiring from Witco in 1996, Professor Mahoney diverted Distinguished Alumnus Award from the University of his energies to developing the next generation of leadership Massachusetts, and the 2006 Columbia University in science and industry. Professor Mahoney was a longtime School of Business Leadership Award. He is the recipient adjunct faculty member in the UMass Chemistry Department. of the 2009 Henry L. Shattuck Boston City Champion He taught a highly successful seminar series entitled “The Award and the 2011 USS Constitution Museum’s Charles Business of Science: Contemporary Practices” for several Francis Adams Award for public service. years. Through this seminar series, students were introduced to topics in the management of science and technology by In February 2014, Mahoney was named the “most- speakers from the business management communities. admired CEO of a small or mid-sized company in Professor Mahoney also chaired the Natural Sciences and Mathematics Advisory Council. In recognition of his William E. -

Nobel Workshop DAY 1 Organized by the Royal Swedish Academy of Sciences and the Chalmers Life Science Engineering Area of Advance
Nobel Workshop DAY 1 Organized by The Royal Swedish Academy of Sciences and the Chalmers Life Science Engineering Area of Advance Date: Monday May 4th, 2015 Topic: MOLECULES IN LIFE SCIENCE RESEARCH Chairs: Prof. Jens Nielsen and Prof. Andrew Ewing Venue: RunAn Conference Hall, Chalmers University of Technology, Chalmersplatsen 1, SE-412 58 Göteborg, Sweden 08:35-08:40 Welcome note: Prof Karin Markides, Chalmers President 08:40-08:45 Opening note: Prof Jens Nielsen, Director of Life Science Engineering 08:45-09:25 Prof DAVID LILLEY, University of Dundee, United Kingdom How does RNA act like an enzyme 09:25-10:05 Prof PETER von HIPPEL, University of Oregon, United States The multiple roles of the non-(sequence)-specific binding of genome-regulatory proteins to DNA in vitro and in vivo. 10.05-10:35 Coffee break 10:35-11:15 Prof JENS NIELSEN, Chalmers University of Technology, Sweden Metagenome analysis of the human gut microbiome 11:15-11:55 Prof TOSHIO YANAGIDA, Osaka University, Japan Role of fluctuations for driving bio-molecular machines 11:55-12:55 Lunch break 12:55-13:35 Nobel Laureate ARIEH WARSHEL, University of Southern California, United States Evaluating and using free energy landscapes for biological functions 13:35-14:15 Nobel Laureate MICHAEL LEVITT, Stanford University, United States Solving Large & Difficult Structures with Less Experimental Data 14:15-14:55 Prof. DINA PETRANOVIC, Chalmers University of Technology, Sweden Yeast as a model for human diseases 14:55-15:25 Coffee break 15:25-16:05 Prof RICHARD LERNER, The Scripps -

April 2012 NUCLEUS Web
DED UN 18 O 98 F http://www.nesacs.org N Y O T R E I T H C E N O A E S S S L T A E A C R C I th N S M A 90 Anniversary Issue of The NUCLEUS S E E H C C TI N April 2012 Vol. XC, No. 8 O CA N • AMERI Monthly Meeting Summer Scholar Report 2012 Esselen Award Meeting at Harvard Regioselective Hydroformylation of Nosyl-protected Esselen Award to Professor Bruce Ganem Homoallylic Sulfonamides Ka H. Cheng and Kian L. Tan, Boston College HepatoChem April Historical Events An Interview of HepatoChem President, Marc Bazin in Chemistry By Leopold May, Catholic University of America Synthetic Liver Metabolites An interview with HepatoChem President, Marc Bazin By Michael P. Filosa HepatoChem (http:// (Fig 3). www.hepatochem.com/) HepatoChem has also partnered with Princeton-based is a company co-found - Novatia, http://www.enovatia.com/, a capillary NMR con- ed in 2008 by Marc tract laboratory, to do structure elucidation of metabolites Bazin, formerly of with small amounts of material (0.1-0.5 mg). Pfizer, and Professor Although only a four-person company at this stage, John T. Groves, holder HepatoChem already has numerous clients ranging from of the Hugh Stott Taylor start-ups to big Pharma. “Our clients are very satisfied with Chair of Chemistry at the quality and speed of our service.” Dr. Bazin stated that Princeton University. the business in liver metabolites is $100 million per year HepatoChem was started and their business is expected to grow rapidly for a number in the Groves lab at of years. -
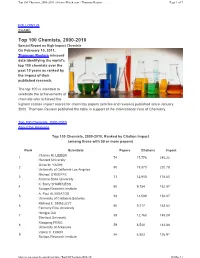
Top 100 Chemists, 2000-2010 - Sciencewatch.Com - Thomson Reuters Page 1 of 7
Top 100 Chemists, 2000-2010 - ScienceWatch.com - Thomson Reuters Page 1 of 7 FOLLOW US SHARE Top 100 Chemists, 2000-2010 Special Report on High-Impact Chemists On February 10, 2011, Thomson Reuters released data identifying the world’s top 100 chemists over the past 10 years as ranked by the impact of their published research. The top 100 is intended to celebrate the achievements of chemists who achieved the highest citation impact scores for chemistry papers (articles and reviews) published since January 2000. Thomson Reuters published the table in support of the International Year of Chemistry. Top 100 Chemists, 2000-2010 About the Analyses Top 100 Chemists, 2000-2010, Ranked by Citation Impact (among those with 50 or more papers) Rank Scientists Papers Citations Impact Charles M. LIEBER 1 74 17,776 240.22 Harvard University Omar M. YAGHI 2 90 19,870 220.78 University of California Los Angeles Michael O’KEEFFE 3 73 12,910 176.85 Arizona State University K. Barry SHARPLESS 4 60 9,754 162.57 Scripps Research Institute A. Paul ALIVISATOS 5 93 14,589 156.87 University of California Berkeley Richard E. SMALLEY† 6 60 9,217 153.62 Formerly Rice University Hongjie DAI 7 88 12,768 145.09 Stanford University Xiaogang PENG 8 59 8,548 144.88 University of Arkansas Valery V. FOKIN 9 54 6,853 126.91 Scripps Research Institute http://sciencewatch.com/dr/sci/misc/Top100Chemists2000-10/ 28.Mar.11 Top 100 Chemists, 2000-2010 - ScienceWatch.com - Thomson Reuters Page 2 of 7 10 Peidong YANG 95 11,167 117.55 [MS 1] University of California Berkeley Benjamin LIST 11 81 8,808 108.74 Max Planck Institute for Coal Research 12 Mark E. -

Office of the President to MEMBERS of the COMMITTEE ON
O1 Office of the President TO MEMBERS OF THE COMMITTEE ON OVERSIGHT OF THE DEPARTMENT OF ENERGY LABORATORIES: ACTION ITEM For Meeting of July 16, 2009 CONFIRMATION OF APPOINTMENT OF A. PAUL ALIVISATOS AS INTERIM DIRECTOR, LAWRENCE BERKELEY NATIONAL LABORATORY Earlier this year, the former Lawrence Berkeley National Laboratory Director, Steven Chu, was confirmed by Congress as the United States Secretary of Energy. Consistent with University of California Standing Order 100.2 (e), the President temporarily appointed A. Paul Alivisatos as Interim Director to facilitate a smooth transition in leadership following Director Chu’s departure. Subsequently, total compensation for Mr. Alivisatos, including a 14 percent stipend, was approved by interim action. (Attachment 2 displays the total compensation approved for this interim appointment.) Consistent with University of California Standing Order 100.2 (e), Regental confirmation of Mr. Alivisatos’ appointment as Interim Laboratory Director, Lawrence Berkeley National Laboratory is requested. RECOMMENDATION The President recommends that the Committee on Oversight of the Department of Energy Laboratories recommend to the Regents confirmation of the appointment of A. Paul Alivisatos as Interim Laboratory Director, Lawrence Berkeley National Laboratory (LBNL). This interim appointment was made pursuant to Standing Order 100.2 (e), and approved by President Yudof on January 21, 2009. The University has requested DOE’s approval of the total compensation and the appointment of the Interim Laboratory Director. DOE has approved the appointment but its approval of the compensation is still pending. As provided under the University’s contract with DOE, any compensation amount approved by the Regents that is over the compensation amount approved by DOE will be paid from the fee earned under the contract COMMITTEE ON -2- O1 OVERSIGHT OF THE DOE LABORATORIES July 16, 2009 BACKGROUND Mr. -

A. PERSONAL DATA Dr. Marcel P. Bruchez Carnegie Mellon
A. PERSONAL DATA Dr. Marcel P. Bruchez Department of Chemistry Carnegie Mellon University Department of Biological Sciences 412 268 9661 (office) Molecular Biosensors and Imaging Center [email protected] http://pathways.mbic.cmu.edu http://bruchez-lab.mbic.cmu.edu ORCID: 0000-0002-7370-4848 ResearcherID: C-2271-2009 Education Nov 1998 Ph.D. in Physical Chemistry from the University of California, Berkeley (Thesis advisor Prof. Paul Alivisatos, Thesis title “Luminescent Semiconductor Nanocrystals—Intermittent Behavior and Use as Fluorescent Biological Probes”) May 1995 B.S. in Chemistry from the Massachusetts Institute of Technology Professional Experience 2011-present Carnegie Mellon University 2014-present (Tenured) Associate Professor, Department of Chemistry & Biological Sciences Director, Molecular Biosensors and Imaging Center 2011-2014 (Untenured) Associate Professor, Department of Chemistry & Biological Sciences Associate Director, Molecular Biosensors and Imaging Center 2011-present Sharp Edge Labs, Inc. Founder and Chief Technology Officer 2011-2006 Carnegie Mellon University Associate Research Professor Department of Chemistry Program Manager for National Technology Center for Networks and Pathways 2005-1998 Quantum Dot Corporation (Acquired by Invitrogen Corp. 10-2005) 1998 Co-Founder and Founding Scientist—Chemistry Division 1998-1995 University of California, Berkeley National Science Foundation Graduate Research Fellow 1995-1991 Massachusetts Institute of Technology Undergraduate Research Associate (Professor Robert G. Griffin) B. PUBLICATION LIST 65 papers published, 28 US Patents Issued, 44 US Published Patent Applications, 1 book edited. Selected manuscripts (h-index of 35, i10-index of 62 per Google Scholar as of 6-1-2016) 1. He, J., Wang, Y., Missinato, M.A., Onuoha, E., Perkins, L.A., Watkins, S.C., St Croix, C.M., Tsang, M., and Bruchez, M.P. -

Connecting with Information at Caltech
Data Points: Connecting with Information at Caltech VOLUME LXXVI, NUMBER 3, FALL 2013 VOLUME LXXVI, NUMBER 3, FALL 2013 2 Caltech on Twitter 3 From the President 4 Random Walk 4UBMBHNJUF$MJNBUF3FDPSET 5IF"SUPG4DJFODF %JUDI%BZ "OE.PSF 9 "WBJMBCMF/PXPO$BMUFDIFEV 10 Seeing Is Knowing BY KATIE NEITH $BMUFDITDJFOUJTUTBOEFOHJOFFSTUBLFJNBHJOHUPUIFOFYUMFWFM QVTIJOH UIFCPVOEBSJFTPGIPXXFTFFUIFQIZTJDBMFOWJSPONFOUBSPVOEVT BOEIPXXFWJTVBMJ[FUIFCVJMEJOHCMPDLTPGUIBUQIZTJDBMXPSME 16 8IBUµTUIF#JH%FBMBCPVU#JH%BUB BY MARCUS Y. WOO "MPPLBUUIFJNQBDUCJHEBUBJTIBWJOHPOTDJFODFBOE IPX$BMUFDIDPNQVUFSTDJFOUJTUTBOEFOHJOFFSTBSF DSFBUJOHUIFUFDIOJRVFTBOEJOGSBTUSVDUVSFUIBUXJMMCF OFFEFEGPSVTUPOBWJHBUFPVSEBUBJOUFOTJWFGVUVSF 24 /BWJHBUJOHUIF#SBJOµT.ZTUFSJFT BY KIMM FESENMAIER $BMUFDISFTFBSDIFST BOEUIF8IJUF)PVTF TBZJUµTUJNF UPQJFDFUPHFUIFSBEZOBNJDNBQPGUIFCSBJO±POFUIBU TIPXTJUTDPNQMFYUSBG¾DLJOHBDSPTTUSJMMJPOTPGOFVSPOBM DPOOFDUJPOT"EESFTTJOHUIJTHSBOEDIBMMFOHFDPVME KVTUCFUIFUFDIOPMPHJDBMNPPOTIPUPGBHFOFSBUJPO 28 &WPMWJOH&EVDBUJPO BY ANDREW ALLAN 5SBJOJOHUIFTDJFOUJTUTBOEFOHJOFFSTPGUIFGVUVSFTUBSUT XJUIUFBDIJOHGPSUIFGVUVSF ON THE COVER: Computational scientist Santiago Lombeyda 32 "MVNOJ*NQBDU(BNF5IFPSZ "VTUFO4UZMF BY THE CALTECH ALUMNI ASSOCIATION created this looping, swirling piece of art (and the more conventional diagram seen on these pages) from the masses of data on gene 35 *O.FNPSJBN interactions coming out of the lab of biologist 'SBODJT)$MBVTFS Barbara Wold, whose work is described on %POBME$PMFT page 21. As part of Caltech’s Center for Advanced Computing Research,