LAKE OPEONGO the Ecology of the Fish Community and of Man’S Effects on It
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skead Road BARK LAKE $16900000.00 +HST
® Skead Road BARK LAKE $16,900,000.00 +HST [email protected] MLS® K19007540 ACREAGE WATERFRONT TAXES PROPERTY TYPE ≈ 1644.46 ACRES ≈ 3.4 km ≈ $19,000 / 2019 MULTI-LOT SUBDIVISION MUNICIPALITY ZONING ACCESS SITE IMPROVEMENTS BARRY’S BAY RU / RT-E20 / MUNICIPAL ROAD VACANT LAND MADAWASKA VALLEY FLOODED AREA Bark Lake, Barry's Bay, Ontario 3.4 kilometers of quality shoreline and 1600 acres of wilderness which abut several thousand acres of crown land. Big western views across this large lake. The lake is 2.5 kilometers wide, 14 kilometers long and is 90 % crown land. Exquisite waterfront estate property to be owned by a group or become the Developer with an incredible opportunity. The subject property consists of 24 individually titled parcels located on and near Bark Lake in Madawaska Valley in Eastern Ontario. The lands form part of a multi-phase, multi-use recreational subdivision. Phase 2 is a draft plan approved with 23 interior lots. Concept plan available for remaining phases or develop your dream! Contact brokerage for further details. Separate offerings are available. East on Hwy 401 from Toronto, North on Hwy 115, east on Highway 7, North on 28 to DIRECTIONS Bancroft, North on 62 to Barry's Bay, Left on 60, left on Skead Rd. Approx. 3.5 hours from Toronto LEGAL DESCRIPTION Contact Brokerage for Detailed Legal Description LISTING BROKERAGE Lake District Realty Corporation, Brokerage - THE WATERFRONT COMPANY™ SALESPERSON Joel Gray, Broker of Record SB COMM 0.5 % Lake District Realty Corporation gathers data from Vendors Disclosure Forms and other public sources for your information. -

The Bonnechere River System W Y LER L IL I M E R Deep River D Lac Lac Mcdonald Sheen Dam Stream/River BAGGS RD Kean H !N Lake U N
! ! ! ! LAMURE RD Lac Esher RB ME A BAL Lac des Îles Head, Clara BANTING DR Y North G R U D T Lac Rouge & Maria Z M à l'Oiseau Lake A N R D BR ON SO N R D Lac LEADER RD Lackey D R Explore the Bonnechere River System W Y LER L IL I M E R Deep River D Lac Lac McDonald Sheen Dam Stream/River BAGGS RD Kean H !n Lake U N D T S R KINGS RD R Perch N D Lake B Bridge Bonnechere River System A )" OT E T AWA K S T Lac à la Truite ! Emm a LAW RD RAILWAYPLANT ST RD Communities Water Lake ! Chalk River S MAIN ST A N D Sturgeon Algonquin Park R County Road D Lake CART IER LAKE RD Young Corry Lake Lake Rorke C DELU RD LOUT THIER Provincial Park Lake HIER R Lac Provincial Highway D Hurdman D R Federal Land Lake E Municipal Maintained Road S C Travers W R L M A O D E E M S D H R R NE R P E ELI E AS I B D N S C L First Nation Reserve T S A I Municipal Seasonal Maintained Road A A A P R N K Y L B R E K RD A IN D K R L E D R D Eganville Cartier M Private Road ID Lake D L E Centre Lake R D Crown Road Municipal Boundary ORANGE RD B P A A RD T D E T S ALIZ S R TOT MESSER TRAIL L E C D H Roads Outside County E R R E S D E N R K A D O L A H R E E E L Lac Tucker K E C C CREST RD Allumette Lac H ickey Sopw ith A Lake U R T R Lake D VERITABLE RD Clemow Lake ARRAS RD D Garrison R ! Petawawa E Laurentian Y M P Lac M R E Hart Ro wan Lake O S Hills S B LVD Lac Noir IA ST TOR Petawawa VIC EAST ST D ! Petawawa ! LAURENTIAN DR R D JAN DR Lac E R Lily Lac S RD E E G NS Bellemare R T A MA T UET T HER O Q R PA PETAWAWA O CHAD ST Grand Lake H RD P P E E TERRACEPROVINCIAL T C Z A Lac du Bois Dur T PARK G A N C W A MURPHY RD A E L R R A N W R N L R LA BARRON SH IR A V H AM BA N L AC L L I E A TIMM RD RIVERPROVINCIAL SS A R A D B I S GUTZMAN RD ! MURPHY RD S D PARK ! L Québec R R T V KE L D T Barron A Black Bay E R L OELKE ST D T EY N Lac URV N Lake S BL D I ACK H R O Coulonge B O CK P AY SA C R BLACK BAY RD P L D K E I RADTKE RD M N R YA DORAN RD KA D B S TRAIL USKIE D KOSS LN RD White RD R R S RIVE Partridge ER SHADY LN M BRUMM RD St. -

AOO Settlement Area Harvest
Wicksteed Lake Lac du Goéland 40 LA SALLE WYSE Lac Smith Marten Lake MCAUSLAN North Spruce Lake Lac du Pin Blanc Poplar LakeHAMMELL GARROWCLARKSON Lac Ramé Lac Bruce Lac Sept Milles Bear Lake OSBORNE POITRAS Lac des Cornes Tilden Lake Map A Lac– Vaucour AOO Settlement Area Harvest Map Lac Saint-Paul Lac des Sables LOCKHART Lac Curières LYMAN NOTMANSTEWART JOCKO EDDY Lac Mosquic Tomiko Lake Lac Nilgaut Lac Barton MERRICK Lac Marin Lac Caugnawana CHARLTONBLYTH Lac Royal Lac des Mocassins MULOCKFRENCHBUTLERANTOINE Lac Maganasipi 41 Lac la Cave Lac Brodtkorb Lac en Croix Lac Gauvin Lac Forbes BEAUCAGE 41 Lac Lamb COMMANDA Lac Resolin Lac Quinn WIDDIFIELD PHELPS Legend OLRIG Lac Murray HWY 17 MATTAWAN Lac à la Tortue Lac McCracken Trout Lake Mattawa Lac Saint-Patrice Lake Talon (lac Talon) Algonquins of Ontario Settlement Area Boundary 42 HWY 17 Lac Dodd Lake Nipissing (lac Nipissing) Rutherglen Lac Duval Lac Wright BONFIELD Lac Schyan FERRIS CALVIN PAPINEAU Deux-Rivières Grand lac des Cèdres Lake Nosbonsing (lac Nosbonsing) Holden Lake Algonquin Park HarvestLac Area Montjoie Stonecliffe Lac Désert Astorville CAMERON CLARA Lac de la Mer Bleue LAUDER Lac Blue Sea Lac Chapleau Wasi Lake (lac Wasi) Rolphton NIPISSING 48 MARIA Harvest Area for Elk BOULTER HEAD CHISHOLM Kiosk BOYD Restoule Lake Lac Galarneau Kioshkokwi Lake ROLPH Deep River Lac Cayamant Lac Marie-Le Franc Commanda Lake PENTLAND FITZGERALD Lac Jim Wildlife Management Unit (WMU) WILKES DEACON Mountain View HIMSWORTH Manitou Lake Carl Wilson Lake Lac McGillivray 47 Cedar Lake BRONSON -

Jeannine-Marie St-Jacques
A Paleolimnological Assessrnent of the Diatom Communities of Lake Opeongo, Ontario, Canada Jeannine-Marie St-Jacques A thesis submitted in canformity with the requirements For the degree of Master of Science, Graduate Department of Botany, University of Toronto O Copyright by Jeannine-Marie StJacques 2000 National Library Bibliothèque nationale 191 du Canada Acquisitions and Acquisitions et Bibliographie Services services bibliographiques 395 Wellington Street 395. nie Wellington Ottawa ON KIA ON4 Ottawa ON K1A ON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distibute or sel1 reproduire, prêter, distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. A Paleolimnological Assessment of the Diatom Communities of Lake Opeongo, Ontario, Canada JeannineMarie St-Jacques Master of Science, 2000 Graduate Department of Botany University of Toronto Abstract Lake Opeongo is an oligotrophic lake in Algonquin Provincial Park, Ontario, Canada (45'42' N, 78O 22' W). Limnological conditions over the past 300 years were tracked using diatoms preserved in the sediments and applying diatom-based transfer functions to infer total phosphorus (TP). -
Renfrew—Nipissing—Pembroke
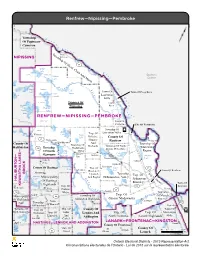
Renfrew—Nipissing—Pembroke Township O t Of Papineau- t a w Cameron a R i v e r T Deux- ow NIPISSING n Rivières sh ip O f H 17 e ad Cedar , C Lake la ra R LISTER DEACON A i v Quebec / Radiant nd M i Québec Lake ar è ia r a w e a P w e a d Hogan ANGLIN t R Lake i e Lake v Town Of Town Of Deep River Lake Travers s e Laurentian Lavieille r Hills O u DICKSON Chalk t District Of a River Nipissing o Dickson Opeongo Lake u Lake a i RENFREW—NIPISSING—PEMBROKE s PRESTON Town Of Petawawa City Of Pembroke AIRY Township Of Aylen Twp. Of Laurentian Valley Whitney Lake Paugh Lake Killaloe, County Of 60 Madawaska Hagarty Renfrew Westmeath And County Of Township Of Township Of Richards Township Of North Whitewater Haliburton 127 Township Madawaska Algona Wilberforce — Of South Valley Region S Bark Barry's Lake Algonquin Kil E laloe — Lake Bay Doré Muskrat K Pikwakanagan N Lake A Lake St. Cobden O K L Peter T Eganville C R A Kamaniskeg Foymount O County Of Hastings Clear 17 U Twp. Of H Lake R Combermere T Lake Town Of Renfrew B Brudenell, I B Maynooth R T L Lyndoch Township w Twp. Of p A Baptiste . A Municipality And Raglan Of Bonnechere Valley O Lake M f H H a Admaston/ W or Of Hastings d t A on a Bromley Town Of K Highlands w Twp. Of 132 Arnprior Wilberforce Birds a Braeside Carlow/ s k a 41 118 Creek Mayo Griffith Township e r Cardiff Twp. -

Lakewoods Drive BARK LAKE $11900000.00
™ Lakewoods Drive BARK LAKE $11,900,000.00 [email protected] MLS® K20001682 LOT SIZE TAXES Water Frontage ZONING ACCESS SURVEY Phase 1 & 2 Plans of ≈1200 Acres ≈ $6615.64 /2019 ≈ 9500 ft Rural Residential Municipal Road Subdivision Bark Lake, Barry's Bay, Ontario 2.5 kilometers of quality shoreline and 1200 acres of wilderness which abut several thousand acres of crown land. Big western views across this large lake. The lake is 2.5 kilometers wide, 14 kilometers long and is 90 % crown land. Exquisite waterfront estate property to be owned by a group or become the Developer with an incredible opportunity. The subject property consists of 10 individually titled parcels located on and near Bark Lake in Madawaska Valley in Eastern Ontario. The lands form part of a multi-phase, multi-use recreational subdivision. Phase 2 is a draft plan approved with 23 interior lots. Concept plan available for remaining phases or develop your dream! Contact brokerage for further details. Separate offerings are available. East on Hwy 401 from Toronto, North on Hwy 115, east on Highway 7, North on 28 to Bancroft, North DIRECTIONS on 62 to Barry's Bay, Left on 60, left on Skead Rd. 3.5 hours. LEGAL DESCRIPTION Contact Brokerage for Full Legal Description LISTING BROKERAGE Lake District Realty Corporation, Brokerage - THE WATERFRONT COMPANY™ SALESPERSONS Joel Gray, SB COMM 1.5% Lake District Realty Corporation gathers data from Vendors Disclosure Forms and other public sources for your information. Purchasers should satisfy themselves on any details they consider important. We encourage you to commission experts to examine a property for you prior to your submitting an Offer of Purchase and Sale. -

Supplemental Report to the Draft Environmental Evaluation Report: Proposed Settlement Lands
Algonquin Land Claim Supplemental Report to the Draft Environmental Evaluation Report: Proposed Settlement Lands Made under the Algonquin Land Claim Declaration Order October 2020 Ontario.ca/page/Algonquin -land-claim-environmental- evaluation-report Cette publication hautement spécialisée Supplemental Report to the Draft Environmental Evaluation Report Proposed Settlement Lands n’est disponible qu’en Anglais conformément au Règlement 671/92, selon lequel il n’est pas obligatoire de la traduire en vertu de la Loi sur les services en français. Pour obtenir des renseignements en français, veuillez communiquer avec le ministère des Richesses naturelles et des Forêts au 1-844-276-9091 ou [email protected]. We are committed to providing accessible customer service https://www.ontario.ca/page/ontario-public-service-ops-accessible-customer- service-policy. If you need any of the information in an alternate format, please contact the Natural Resource Information and Support Centre https://www.ontario.ca/feedback/contact-us or call toll-free: 1-800-667-1940 (Monday to Friday 8:30 am to 5:00 pm E.T. except statutory holidays) or via TTY: 1- 866-686-6072. We will acknowledge emails and online messages within two business days and answer them within 15 business days. Supplemental Report to Draft EER: Proposed Settlement Lands October 26 2020 Table of Contents Part I – Overview ......................................................................................................................................... 1 Partie I – Aperçu ........................................................................................................................................ -

101 Things to Do in the Valley November-December 2020 Edition
101 Things To Do in the Valley NOVEMBER-DECEMEBR ISSUE Subscribe for FREE to this list and get it every two months: [email protected] Download this list at www.travelourbackyard.com ALL SEASON NUMBER 5 NUMBER 9 NUMBER 13 Valley Artisans 20% Off For 2020 Christmas Gifts NUMBER 1 The longest-running artisan co- Beyond Nutrition in Petawawa Looking for a unique Christmas Frozen Meals operative in Ontario. Featuring a is celebrating 20 years in gift for this year? Consider Locally made frozen meals are unique selection of locally business. Come in and receive purchasing a foot of runway for available from Griffith Farm & handcrafted fine art and fun gifts 20% off all purchases for the someone on your list. This is a Market. 24 meal choices from from over 30 artists and crafters. remainder of 2020. Boost fundraiser to repave the 5,000- beef stew and cabbage rolls to Jewelry, woodworking, custom immunity, treat anxiety and foot runway at the Pembroke & soups and roast pork. Made with razors, books, drums, glasswork, more. Plenty of products to help Area Airport. $250 per foot. real ingredients and no fillers. greeting cards, silk, weaving, keep you healthy this fall and 100% charitable receipt Fully cooked and easy to reheat knitting, sewing, painting and winter. 3468 Petawawa provided. in the microwave or oven. drawing and cut paperwork, Boulevard in Petawawa. Phone/Text: 613-312-7824 Phone: 613-401-6956 photography, pottery and [email protected] marketing@flycyta.ca www.griffithfarm.ca skincare products. NUMBER 10 NUMBER 14 NUMBER 2 33373 Highway 17, Deep River Visit Runge Stationers Snowmobile Draw MPP Yakabuski www.ValleyArtisans.com 2021 planners, stationery, Winter Snowmobile Package. -

INSIDE RENFREW COUNTY Stories of Entrepreneurship and Business Success in the Ottawa Valley
INSIDE RENFREW COUNTY Stories of entrepreneurship and business success in the Ottawa Valley CONTENTS: Investment opportunities P.2 High tech P.4 Quality of life P.6 Key contacts P.8 INSIDE RENFREW COUNTY Lianne Ing, vice-president of Chalk River-based Bubble Technology Industries, speaks with Prime Minister Justin Trudeau. Investment opportunities Land availability, workforce and low operating COMMERCIAL REAL ESTATE costs open business opportunities Renfrew County is home to a diverse economic base of small and medium- ATURE FIRST ATTRACTED that makes television programs sized businesses that includes BRENDAN MARK to Renfrew about adventure travel and the manufacturing facilities, tourism NCounty’s Whitewater Region. outdoors, including series on operators, nuclear science and A former professional kayaker, he hunting, fishing and the best hiking technology, aerospace and defence quickly developed a passion for trails in the world. industries, among others. the region’s rivers. “We love the Filming in far-flung locations They all benefit from a healthy outdoors and we love the access to around the world means some supply of well-priced industrial land the outdoors that (the area) gives us,” Heliconia employees work and buildings, low development he says. remotely. However, the company’s charges, a skilled labour force, While Mr. Mark can indulge in headquarters are firmly rooted in strong transportation links, his personal passions virtually at his Renfrew County. proximity to major markets, an doorstep, his business is decidedly “The reason we’re able to do that unparalleled quality of life and a international. Being located in is the foresight that the region had in municipal economic development Renfrew County gives him the best investing in high-speed internet. -

Reports on the Algonquin National Park of Ontario for the Year 1893
: REPORTS ON THB ALGONQUIN NATIONAL PARK OF ONTARIO FOR THE YEAR 1893. PRINTED BY ORDER OF THE LEGISLATIVE ASSEMBLY. TORONTO WARWICK BROS. & RUTTER, PRINTERS, 68 and 70 FRONT ST. WEST 1894. z REPORTS ON THE ALGONQUIN NATIONAL PARK OF ONTARIO FOR THE YEAR 1893. FEINTED BY ORDER OF THE LEGISLATIVE ASSEMBLY. _ „ TORONTO: WARWICK BROS. & RUTTER, PRINTERS, &o., 6 08 AND 70 FRONT STREET WEST. 1894. CONTENTS. Page. Letter of Transmission 5 Report of Chief Ranger Thorns on 7 Report of Mr. James Wilson 11 The Algonquin National Park Act 21 ,1~V ^. A VI Digitized by the Internet Archive in 2013 http://archive.org/details/reportsonalgonquOOonta REPORTS ON THE ALGONQUIN NATIONAL PARK OF ONTARIO FOR THE YEAR 1893. To His Honor the Honorable George Airey Kirkpatrick, Lieutenant- Governor of the Province of Ontario : Sir, — I beg to submit herewith, for the information of Your Honor and the Legis- lative Assembly, the following reports, one by Mr. Peter Thomson, Chief Ranger, and the other by Mr. James Wilson, Superintendent of Queen Victoria Niagara Falls Park, in connection with The Algonquin National Park of Ontario. Mr. Thomson's report gives a brief account of the preparatory and other work done in the Park since it was set apait by Act of the Legislative Assembly last year. Mr. Wilson, who has had long experience as Superintendent of the Queen Victoria Park at Niagara Falls, was requested to visit the Park in the autumn of last year and make a report thereon. In his report Mr. Wilson gives an interesting description of portions of the Park traversed by the principal waterways, and also makes a number of suggestions as to its management and administration. -

Launch of MV Family Health Team Marks Major Advance in Access to Local Healthcare
FREE INSIDE… The Current review its first year p.3 Heritage Photo: St. Joseph’s School p.7 Rescue drama on Siberia Road p.9 FRIDAY, JANUARY 18, 2019 Launch of MV Family Health Team marks major advance in access to local healthcare Cutting the ribbon to mark the Grand Opening (from left): Renfrew-Nipissing-Pembroke MPP John Yakabuski, MVFHT Board Chair Dr. Alex Atfield, MVFHT Lead Physician Dr. Jason Malinowski, BLR Mayor Sheldon Keller, MV Mayor Kim Love, KHR Mayor Janice Visneskie- Moore, Champlain LHIN Board Chair Jean-Pierre Boisclair (holding scissors), MVFHT Executive Director Susan Farrar, NAW Mayor James Brose, SA Mayor Jane Dumas, Champlain LHIN Sub-Region Director Kevin Barclay, MVFHT Office Administrator Rosa Etmanskie. DANIELLE PAUL who told the crowd how far they had dar, because they came back to us a few MADAWASKA VALLEY come in the past year. He pointed out months later and said, ‘The projects we that the Madawaska Valley FHT is one have in mind didn’t pass muster with The patient waiting area at the St. of the first Family Health Teams (FHTs) the Ministry. We’ve looked at our data Francis Health Centre in Barry’s Bay created in Ontario since 2012. He said, again. We drilled down to the commu- was crowded on January 11, 2019 “We’re really lucky to be at the begin- nity level and it turns out you’re right with representatives of provincial and ning of, hopefully, a new wave of FHTs – you are in high need. Can we work to- municipal government, local and re- in Ontario. -

My Own Private River
Spring 1990 Vol. 17 No.1 Quarterly Journal of the Wilderness Canoe Association MY OWN PRIVATE RIVER Bob McCoubrie Seeking the joys and satisfactions of canoeing untravelled BATHTUB FALLS territory in the near North, I began exploring an area in the vicinity of North Caribou Lake, northwest Ontario, with Our first journey up the Nango (1980) rewarded us with groups of boys in 1979. In some areas we found ancient unending delights of discovery. By early afternoon of the portages, long unused, and it was hard, satisfying work to first day, we came to rapids that required our first full reopen them. Other areas yielded routes never used, and we portage. One section of the narrowed river resembled a huge cut the first portages there. jacuzzi with water boiling in violently at one end. The safe In 1980, in order to reach the road extending north from swimming and diving possibilities at that spot and the time Pickle Lake, we travelled up the 62-km Nango River. We that it was going to take to cut a 400-m portage were good found no evidence of its ever having been travelled, and the excuses to make camp immediately. We quickly discovered uniqueness of finding and canoeing a whole never-travelled the various antics we could do for the most fun in the wild river, and the pleasures, satisfactions, and values derived currents in our newfound "Bathtub Falls." from it caused me to explore it five times. Arrival at Bathtub our second year (1981) held no joy. Early in our trip we had noted fires to the south of North Nastawgan Spring 1990 Caribou Lake.