Giant Cell Tumor of Bone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View Presentation Notes
When is a musculoskeletal condition a tumor? Recognizing common bone and soft tissue tumors Christian M. Ogilvie, MD Assistant Professor of Orthopaedic Surgery University of Pennsylvania University of Pennsylvania Department of Orthopaedic Surgery Purpose • Recognize that tumors can present in the extremities of patients treated by athletic trainers • Know that tumors may present as a lump, pain or both • Become familiar with some bone and soft tissue tumors University of Pennsylvania Department of Orthopaedic Surgery Summary • Introduction – Pain – Lump • Bone tumors – Malignant – Benign • Soft tissue tumors – Malignant – Benign University of Pennsylvania Department of Orthopaedic Surgery Summary • Presentation • Imaging • History • Similar conditions –Injury University of Pennsylvania Department of Orthopaedic Surgery Introduction •Connective tissue tumors -Bone -Cartilage -Muscle -Fat -Synovium (lining of joints, tendons & bursae) -Nerve -Vessels •Malignant (cancerous): sarcoma •Benign University of Pennsylvania Department of Orthopaedic Surgery Introduction: Pain • Malignant bone tumors: usually • Benign bone tumors: some types • Malignant soft tissue tumors: not until large • Benign soft tissue tumors: some types University of Pennsylvania Department of Orthopaedic Surgery Introduction: Pain • Bone tumors – Not necessarily activity related – May be worse at night – Absence of trauma, mild trauma or remote trauma • Watch for referred patterns – Knee pain for hip problem – Arm and leg pains in spine lesions University of Pennsylvania -

A Rare Case of Chondromyxoid Fibroma of the Scapula Jay B
A Case Report & Literature Review A Rare Case of Chondromyxoid Fibroma of the Scapula Jay B. Jani, MD, Kathleen S. Beebe, MD, Meera Hameed, MD, and Joseph Benevenia, MD hondromyxoid fibroma (CMF) is a rare benign Plain radiography (Figures 1A, 1B) and computed tumor, apparently derived from cartilage-forming tomography (CT) scan (Figure 2) revealed an expansile connective tissue. The name is highly descriptive lesion of the right scapula with central calcification sug- of this distinctive tumor and has gained accep- gesting chondroid-type matrix. There was some thinning Ctance.1 The entity was first described in 1948 by Jaffe and of the cortex but no obvious cortical breach or associated Lichtenstein,2 who presented 8 cases and emphasized the soft-tissue mass. MRI (Figure 3) revealed a 5×3×2.5- danger of mistaking this benign neoplasm for a malignant cm expansile lesion involving the inferior border of the lesion, chondrosarcoma in particular. Approximately two scapula. T2-weighted images showed a heterogeneous thirds of the recorded cases of this tumor have been in the mass with bright signal intensity. There was considerable long tubular bones and one third in the proximal tibia.1,3,4 A edema in the teres minor and subscapularis muscle bel- scapular origin of this tumor is exceedingly rare.1,5-10 lies. No fluid–fluid levels were seen. Additional workup We report the case of a 13-year-old girl with chondro- included a chest CT scan and a whole-body bone scan. myxoid fibroma of the scapula. This case is of interest The bone scan revealed increased focal uptake to the right because of the rarity and unusual location of the tumor. -

A Case of Thoracic Vertebral Chondroblastoma, Treated with 3-D Image Guided Resection and Reconstruction
KISEP J Korean Neurosurg Soc 37 : 154-156, 2005 Case Report A Case of Thoracic Vertebral Chondroblastoma, Treated with 3-D Image Guided Resection and Reconstruction Yoon Ho Lee, M.D., Dong Ah Shin, M.D., Keung Nyun Kim, M.D., Do Heum Yoon, M.D. Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea We present a case of chondroblastoma in the thoracic vertebra. A 40-year-old patient with upper back pain and lower extremity weakness was admitted to our clinic. On neurological examination, the patient exhibited lower extremity spastic paraparesis. Magnetic resonance imaging revealed a mass infiltrating the 7th thoracic vertebra and its adjacent structures with concomitant compression of the epidural space. After right upper lung tuberculoma was resected through the transthoracic approach, T7 total corpectomy was done with anterior stabilization using a MESH cage and T7 rib bone graft. Two weeks after the first operation, remained part of vertebra was removed and posterior stabilization was performed using a pedicle screw fixation and cross linkage bar with the assistance of the navigation system. The final pathologic diagnosis of the vertebral lesion was benign chondroblastoma. KEY WORDS : Chondroblastoma·Thoracic vertebra·Spine tumor. Introduction hondrobalsoma is a benign C bone tumor arising most often in the epiphysis of long bones. The vertebra is a rare pri- mary site of origin for chondro- blastomas. We report a 40-years old woman with chondroblastoma A B C of the 7th thoracic vertebral body and the adjacent structures. In Fig. 1. Preoperative T1-weighted enhanced axial(A) and sagittal(B) magnetic resonance images and computed tomography(C) scan demonstrating tumor involvement of the T7 vertebra with spinal addition, the use of an intraop- cord compression. -

Pelvic Chondroblastoma in an Adolescent. New Treatment Approach
www.medigraphic.org.mx Acta Ortopédica Mexicana 2011; 25(6): Nov.-Dec: 388-394 Clinical case Pelvic chondroblastoma in an adolescent. New treatment approach Rico-Martínez G,* Linares-González L,** Delgado-Cedillo E,** Cerrada-Moreno L,*** Clara-Altamirano M,*** Pichardo-Bahena R**** National Rehabilitation Institute. Mexico, D.F. ABSTRACT. Surgical management of tumors RESUMEN. Los tumores que están localizados located in the spine and the pelvis involves greater en la columna vertebral y en la pelvis tienen aso- diffi culty. Moreover, these tumors are usually very ciada una mayor difi cultad para el manejo quirúr- large and vascularized. Preoperative embolization gico. Además, estos tumores son habitualmente de of the internal iliac artery is a relatively safe pro- gran tamaño y ricamente vascularizados. La em- cedure that may reduce the risk of bleeding and lo- bolización prequirúrgica arterial de la arteria ilía- cal recurrence in the case of benign tumors. Chon- ca interna tiene la bondad de ser un procedimiento droblastoma is a tumor that is rarely located in the relativamente seguro que puede reducir el riesgo pelvis; its more frequent location is the triradiate de sangrado y de recidiva local en tumores benig- cartilage. We describe a case of a chondroblasto- nos. El condroblastoma es un tumor raro en la pel- ma with a relapsing aneurysmal cystic component vis y la localización más frecuente es en el cartílago in the acetabulum of an adolescent patient. Treat- trirradiado. Se presenta un caso de condroblasto- ment consisted of embolization of the internal iliac ma con componente quístico aneurismático recidi- artery, fl uid hyperthermia, hydrogen peroxide and vante en el acetábulo de un paciente adolescente. -

Chondroblastoma: a Rare Cause of Femoral Neck Fracture in a Teenager Michael D
A Case Report & Literature Review Chondroblastoma: A Rare Cause of Femoral Neck Fracture in a Teenager Michael D. Paloski, DO, Michael J. Griesser, MD, Mark E. Jacobson, MD, and Thomas J. Scharschmidt, MD chanter apophysis, review the literature, and present Abstract learning points for this diagnosis and treatment. Chondroblastomas usually present in the epiphyseal The patient provided written informed consent for region of bones in skeletally immature patients. These print and electronic publication of this case report. uncommon, benign tumors are usually treated with curet- tage and use of a bone-void filler. ASE EPORT Here we report a case of a hip fracture secondary to C R an underlying chondroblastoma in a 19-year-old woman. The patient was an otherwise healthy 19-year-old Open biopsy with intraoperative frozen section pointed white woman who presented to the emergency toward a diagnosis of chondroblastoma. Extended curet- department with the chief report of right hip pain, tage was performed, followed by cryotherapy with a liquid and inability to ambulate after slipping on ice and nitrogen gun and filling of the defect with calcium phos- falling on her left side from standing height. She phate bone substitute. The femoral neck fracture was stated she had a 3-year history of intermittent stabilized with a sliding hip screw construct. The patient right hip pain before this incident. At that time, her progressed well and continued to regain functional sta- primary care physician had worked up her initial tus. A final pathology report confirmed the lesion to be a symptoms with radiographs, which were reported chondroblastoma. -

Evaluation of Some Histochemical and Immunohistochemical Criteria of Round Cell Tumors of Bone Eman M
Original article 21 Evaluation of some histochemical and immunohistochemical criteria of round cell tumors of bone Eman M. S. Muhammada, Zeinab H. El-badawia, Sana S. Krooshc and Hassan H. Noamanb Departments of aPathology, bOrthopedic Surgery, Background/aims Faculty of Medicine, University of Sohag, Sohag and cDepartment of Pathology, Faculty of Medicine, The differential diagnosis of round cell tumors of bone (RCTB), Ewing sarcoma, small- University of Assiut, 82524 Assiut, Egypt cell osteosarcoma, mesenchymal chondrosarcoma, osteoblastoma, chondroblastoma, Correspondence to Eman M. S. Muhammad, primary bone lymphoma, and multiple myeloma still remains a challenge. Given the Department of Pathology, Faculty of Medicine, significant differences in treatment, an accurate diagnosis is crucial. This study aimed University of Sohag, Sohag, Egypt Tel:+ 02 093 460 2963; e-mail: [email protected] to evaluate some histochemical and immunohistochemical criteria of RCTB. Participants and methods Received 29 January 2012 Accepted 6 March 2012 Periodic acid-Schiff (PAS), CD99, CD138, osteocalcin, and leukocyte common antigen (LCA) were evaluated in 113 patients with RCTB. Journal of the Arab Society for Medical Research 2012, 7:21–32 Results PAS was positive in neoplastic cells of all Ewing sarcomas, 27% of osteosarcomas, 92% of chondrosarcomas, all osteoblastomas and chondroblastomas, and the osteoid tissue of all osteosarcomas and osteoblastomas. CD99 was positive in all Ewing sarcomas, in 11, 4, and 11% of osteoblastomas, multiple myelomas, and bone lymphomas, respectively. CD99 was higher in Ewing sarcoma than in other RCTB (Po0.0001). Osteocalcin was positive in neoplastic cells of all osteosarcomas, osteoblastomas, and 20% of chondroblastomas, 84, and 78% of osteoid of osteosarcomas and osteoblastomas, respectively. -

Chondroblastoma: an Unusual Cause of Shoulder Pain in Adolescence
Lambert, J et al 2016 Chondroblastoma: An Unusual Cause Of Shoulder Pain In Adolescence. Journal of the Belgian Society of Radiology, 100(1): 16, pp. 1–3, DOI: http://dx.doi.org/10.5334/jbr-btr.1027 CASE REPORT Chondroblastoma: An Unusual Cause Of Shoulder Pain In Adolescence Julie Lambert*, Tom Verstraeten† and Koen Mermuys‡ Chondroblastoma is a rare benign bone tumor, most often localized in the epiphysis of long bones. We report a case of atraumatic shoulder pain in a 17-year old soccer player. This chondroblastoma case dem- onstrates the difficult differentiation of chondroblastoma from giant cell tumor and clear cell chondrosar- coma and highlights possible pitfalls and clinical importance. Keywords: Chondroblastoma; Shoulder pain; Radiography; Magnetic resonance imaging; Multidetector computed tomography A 17-year-old athletic male presented to the orthopedic center for consultation regarding left shoulder pain last- ing more than two months. There was no recent trauma. Clinical examination was unremarkable, with a normal mobility of the shoulder joint. Ultrasound examination of the shoulder was normal. There was no fever or any other sign of systemic illness and laboratory findings were normal. A single corticoid injection was given, which did not result in any pain relief. Subsequently, magnetic reso- nance (MR) arthrography, performed for the detection of ligamentous or labral injury, revealed a bone lesion located at the epiphysis of the humeral head (Figure 1). The location and morphology of this lesion, as well as the age of the patient, were suggestive for chondroblas- toma. However, the hyper-intense signal intensity on the T2-weighted images was a typical for this diagnosis. -
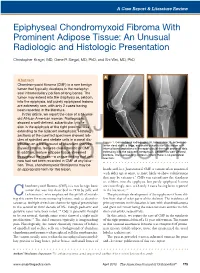
Epiphyseal Chondromyxoid Fibroma with Prominent Adipose Tissue: an Unusual Radiologic and Histologic Presentation
A Case Report & Literature Review Epiphyseal Chondromyxoid Fibroma With Prominent Adipose Tissue: An Unusual Radiologic and Histologic Presentation Christopher Kragel, MD, Gene P. Siegal, MD, PhD, and Shi Wei, MD, PhD A B Abstract Chondromyxoid fibroma (CMF) is a rare benign tumor that typically develops in the metaphy- seal intramedullary portion of long bones. The tumor may extend into the diaphysis or, seldom, into the epiphysis, but purely epiphyseal lesions are extremely rare, with only 2 cases having been reported in the literature. In this article, we report the case of a 51-year- old African American woman. Radiographs showed a well-defined, subarticular lytic le- sion in the epiphysis of the right proximal tibia extending to the adjacent metaphysis.AJO Histologic sections of the curetted specimen showed lob- ules of spindled and stellate cells in a zonal dis- Figure 1. Conventional radiographs (A, lateral view; B, anteropos- tribution on a background of abundant chondro- terior view) show a large, expansile subarticular lytic lesion with myxoid stroma, features characteristic of CMF. internal bony septations in the epiphysis of the right proximal tibia In addition, mature adipose tissue streamed extending into the adjacent metaphysis. Lesion has well-defined borders. The surrounding cortex is intact; there is no periosteal throughout the lesion—a unique finding that until reaction. DOnow had not been recorded NOT in CMF at any loca- COPY tion. Thus, chondromyxoid fibrolipoma may be an appropriate term for this lesion. hands and -

Peripheral Giant Cell Granuloma: a Case Report
Open Access Journal of Dental Applications A Austin Full Text Article Publishing Group Case Report Peripheral Giant Cell Granuloma: a Case Report Abhishek Kumar1*, Varun Pratap Singh2 and Priyanka Shah3 Abstract 1Assistant Professor, Department of Pedodontics & Peripheral giant cell granuloma is one of the reactive hyperplastic lesions of Preventive Dentistry, College of Dental Surgery, BP the oral cavity, which originates from the periosteum or periodontal membrane Koirala Institute of Health Sciences, Dharan, Nepal following local irritation or chronic trauma. This article reports a case of 2Associate Professor & Head, Department of peripheral giant cell granuloma in an 11 years old female patient. The patient’s Orthodontics, Nobel Medical College & Teaching mother had reported to the Department of Pedodontics and Preventive Dentistry Hospital, Biratnagar, Nepal. with a chief complaint of swelling in the right back region of upper jaw since four 3Junior Resident, Department of Pedodontics & months. Radiographically, (CT scan PNS, maxilla, mandible) revealed ground Preventive Dentistry, College of Dental Surgery, BP glass appearance of alveolar process of right maxilla with bony outgrowth along Koirala Institute of Health Sciences, Dharan, Nepal with associated soft tissue component arising from the inner surface of alveolar *Corresponding author: Abhishek Kumar, Assistant process of right maxilla.The lesion was excised and sent for histopathologic Professor, Department of Pedodontics & Preventive examination which confirmed the diagnosis of Peripheral Giant Cell Granuloma. Dentistry, College of Dental Surgery, BP Koirala Institute The 6-month clinical follow-up revealed uneventful soft issue healing. Early and of Health Sciences, Dharan, Nepal, Email: kumar.pedo@ definite diagnosis correlating clinical, radiologic and histopathologic examination gmail.com is important for conservative management of such lesion thus eliminating potential risk to adjacent hard tissue structures. -

Fresh Tissue Multi-Omics Profiling Reveals Immune Classification And
Author Manuscript Published OnlineFirst on August 23, 2021; DOI: 10.1158/1078-0432.CCR-21-1893 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. 1 Fresh tissue multi-omics profiling reveals immune classification and 2 suggests immunotherapy candidates of conventional chondrosarcoma 3 Binghao Li,1,2,3,† Guoqi Li,2,3,† Xiaobo Yan,1,3,† Dan Zhu,4 Patrick P. Lin,5 Zenan Wang,2,3 Hao 4 Qu,1 Xuexin He,1,6 Yanbiao Fu,1,7 Xiuliang Zhu,8 Peng Lin,1,2 Jiangnan Zhang,1 Xiaoya Li,1 Hui 5 Dai,9 Huabiao Chen,10 Mark C. Poznansky,10 Nong Lin,1,3* and Zhaoming Ye1,2,3,*,# 6 Authors’ affiliations: 7 1. Orthopaedic Oncology Services, Department of Orthopaedics, The Second Affiliated Hospital 8 of Zhejiang University School of Medicine, Hangzhou 310009, China 9 2. Orthopaedic Research Institute, Zhejiang University, Hangzhou 310009, China 10 3. Key Laboratory of Motor System Disease Research and Precision Therapy of Zhejiang 11 Province, Hangzhou 310009, China 12 4. Division of Mass Cytometry, PLTTECH Institute, Hangzhou 310009, China 13 5. Department of Orthopaedic Oncology, Division of Surgery, The University of Texas MD 14 Anderson Cancer Center, Houston, TX 77230, USA 15 6. Department of Medical Oncology, The Second Affiliated Hospital of Zhejiang University 16 School of Medicine, Hangzhou 310009, China 17 7. Department of Pathology, The Second Affiliated Hospital of Zhejiang University School of 18 Medicine, Hangzhou 310009, China 19 8. Department of Radiology, The Second Affiliated Hospital of Zhejiang University School of 20 Medicine, Hangzhou 310009, China 21 9. -

Ewing Sarcoma of Proximal Tibial Epiphysis: an Unusual Presentation with Review of Literature
Original Research Article DOI: 10.18231/2394-6792.2018.0058 Ewing sarcoma of proximal tibial epiphysis: An unusual presentation with review of literature Ruchi Sinha1, Iffat Jamal2,*, Amar Kumar3, Anup Kumar4 1,4Addtional Professor, 2Senior Resident, 3Tutor, 1-3Dept. of Pathology/Lab Medicine, 4Dept. of Orthopaedics, All India Institute of Medical Sciences, (AIIMS) Patna, Bihar, India *Corresponding Author: Email: [email protected] Received: 17th July, 2017 Accepted: 11th November, 2017 Abstract Ewing’s sarcoma/Primitive neuroectodermal tumor (ES/PNET) of bone is a rare childhood tumor most commonly located in the metadiaphysis. It is more commonly seen in the appendicular skeleton and typically involves femur, tibia, humerus or fibula. Within the long bones, the tumor is always metaphyseal or diaphyseal (mid-diaphysis: 33%, metadiaphysis: 44%, metaphyseal: 15%). Rarely 1%-2% of ES may involve epiphysis. In skeletally immature patients, lesions of the epiphysis are rarely malignant, with the most common diagnosis benign chondroblastoma. We report a case of ES of the tibial epiphysis in a 16 year old boy presenting with complaints of pain around right knee joint for six months associated with fever and restricted mobility of knee joint. Magnetic resonance imaging (MRI) of the patient’s right knee revealed a large lesion in the centre of upper tibial epiphysis showing hypointense signal on T1 & T2W1 extending from the articular surface through the epiphyseal plate into the upper metaphyseal region. MRI findings suggested an infective pathology with differential diagnosis of Brodie’s abscess and chondroblastoma. Based on the imaging characteristics, the patient’s age and the lesion’s location a preliminary diagnosis of chondroblastoma was made. -

The Review Literature About Chondroblastoma with a Case On
Hong Kong Journal of Orthopaedic Research 2020; 3(3): 68-73 Case Report The Review literature about Chondroblastoma with a case Hong Kong J Orthop Res ISSN (e): 2663-8231 on Talus bone ISSN (p): 2663-8223 2020; 3(3): 68-73 Sayed Abdulla Jami1*, Siam Al Mobarak2*, M M Sohel Tanvir1, Syma Nosin2 © 2020, All rights reserved 1 Department of Spinal Surgery, General Hospital of Ningxia Medical University, Ningxia Medical University 804 www.hkorthopaedicjournal.com Shengli Street, Xingqing District, Yinchuan, 750004, Ningxia, People’s Republic of China 2 First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China Abstract Background: Chondroblastomas are rare bone tumors, which are the cartilaginous origin and account for nearly 1% of all primary bone tumors. It can affect people of all ages. Chondroblastomas arising in the talus are very rare and are known to have less aggressive behavior. Methods: 11 authors, a total of 572 patient data with our talus case, were analyzed for this review literature. Here is the mentioning of epidemiological and clinical information, radiological details, surgery type, adjuvants types, genders, follow-up years, recurrence, affected areas, death rates, successful outcome, MSTS functionality scores, and survival rates by analyzing all data. Result: Chondroblastoma was treated by curettage and bone grafting during surgical procedures. This procedure is curative in 90% of cases. There was no recurrence in this study case of the talus. Also, the MSTS functionality score for CB averages 90% more in most of the author’s literature, and 0% death rate was reported. Conclusion: We believe that chondroblastoma of the talus bone should be considered among the differential diagnosis whenever a neoplastic cause is being considered as the underlying pathologic entity.