Willow Lignin Oxidation and Depolymerization Under Low Cost
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal Name !"#$%&'(#)*'
ChemComm 2?%&*-;(@$.-;'+(A-%B0 !Page 4 of 8 !"#$%&'()&*+ ,-.+(./-01(2341(5675689:;6<<66666< ===7$0;7"$>:<<<<<< !"#$%&'(#)*' Self-Assembly in the Electrical Double Layer of Ionic Liquids Susan Perkin,*a Lorna Crowhurst,b Heiko Niedermeyer, b Tom Welton, b Alexander M. Smith a and Nitya Nand Gosvamia Received (in XXX, XXX) Xth XXXXXXXXX 20XX, Accepted Xth XXXXXXXXX 20XX 5 DOI: 10.1039/b000000x We have studied the structure of two ionic liquids confined layering structure is observed for the two ILs, despite their between negatively charged mica sheets. Both liquids exhibit 50 similar chemical structures, which differ only in the length of interfacial layering, however the repeat distance is hydrocarbon chain on the cation. We propose that the more dramatically different for the two liquids. Our results suggest amphiphilic nature of the [C6C1im] cation causes self-assembly 10 a transition from alternating cation-anion monolayers to tail- into tail-to-tail cation bilayers at the mica surface, driven by the to-tail cation bilayers when the length of the cation need to sequester the hydrocarbon chains away from the ionic hydrocarbon chain is increased. 55 regions. Many applications of ionic liquids (ILs) involve their use as electrolytes in electrochemical applications such as solar cells and 15 electrical double-layer capacitors (EDLCs, also called supercapacitors)1, 2. Currently there is relatively little understanding of the interfacial behaviour of ILs at charged surfaces, although recent experimental and theoretical work have revealed that the electrical double layer in IL is dramatically 20 different to that in dilute electrolyte solutions. Experiments using Figure 1: Chemical structures and approximate dimensions in nm, a Surface Force Apparatus (SFA)3, 4 and AFM5 suggest that next calculated from the van der Waals radii, of the ions used in this study. -

Ionic Liquids in Bio-Refining Synthesis and Applications
Ionic liquids in bio-refining Synthesis and applications John Gräsvik Doctoral thesis Department of Chemistry Umeå University Umeå, Sweden 2013 Copyright © John Gräsvik ISBN: 978-91-7459-655-7 Omslagsbild: Föryngringsyta i Lumiovaara utanför Keräntöjärvi Electronic version available at http://umu.diva-portal.org/ Printed by: VMC-KBC, Umeå University Umeå, Sweden 2013 Abstract Fossil fuel recourses are not limitless so alternative renewable recourses are needed to fill the void that inevitably will be created once the supplies of this recourse start do dwindle. Biomass has the potential to fill this void. Today only a small part of the world annual production of biomass is utilized by humankind, while the rest is allowed to decay naturally. To utilize this renewable recourse in the production of fuel and chemicals, the so called bio- refineries specialized in fractionation and making use of all component of the biomass are needed. Ionic liquids could aid in this task. Ionic liquids (ILs) have shown great potential in the field of biomass processing in general and in the pretreatment of (ligno)-cellulose in particular. However, a few things need to be addressed before any large-scale processing can be considered: Finding new routes for IL synthesis that make “on-site” production possible; Investigation into the challenges facing IL pretreatment of (ligno)-cellulose such as possible depolymerization of cellulosic material during the pretreatment and investigating what influence different ILs have on the pretreatment of cellulosic material by methods like enzymatic hydrolysis. This work aims to address these issues and will present a route for IL synthesis making use of alcohols and carboxylic acids both commonly found in a biorefinery. -

MSDG Schedule
Summer Research Meeting 24 — 26 July, Downing College, Cambridge Schedule and presentations Meeting Schedule Tuesday 24th Wednesday 25th Thursday 26th 08:30 — 09:00 Tea & coffee Tea & coffee 09:00 — 09:30 The Niccolo Le Brun FRAY LECTURE 09:30 — 10:00 Derek Fray Geir Martin Haarberg 10:00 — 10:30 Andrew Watson Leigh Aldous 10:30 — 11:00 Tea & coffee Tea & coffee 11:00 — 11:30 David Rodriguez Xianbo Jin 11:30 — 12:00 Maurizio Celentano George Chen 12:00 — 12:30 Ali Kamali Registration LUNCH LUNCH 12:30 — 13:30 LUNCH Committee meeting Conference close Chair’s welcome 13:45 13:30 — 14:00 Yumi Katasho 14:00 — 14:30 Charles Denbow Nicholas Gathergood 14:30 — 15:00 Ian Scott 15:00 — 15:30 Rasmus Fehrmann 15:30 — 15:45 Tea & coffee Tea & coffee 15:45 — 16:15 Espen Olsen 16:15 — 16:45 Stuart Mucklejohn Museum visit 16:45 — 17:15 Ian Mellor 17:15 — 17:45 Drinks reception Drinks reception 18:00 — 19:00 West Lodge Grace Howard Room Conference dinner Gala dinner 19:00 — 22:00 Maitland Room Speaker Tom Welton Welcome to Cambridge The Molten Salts Discussion Group has the pleasure of welcoming you to Downing College. We have a full schedule of talks on all areas of molten salts and ionic liquids. This year we inaugurate a new named lecture, the Fray Lecture, and the inaugural speaker will be Prof Derek Fray FRS FRAEng. We hope you enjoy your stay in Cambridge. MSDG Committee Getting to Cambridge Train Cambridge is well connected by train. -

Chemistry Update Newsletter 292, 18 December 2017
Chemistry Update Newsletter 292, 18 December 2017 Inside this Issue North Sea Water and Recycled Metal Combined to 2-3 Calendar of Events Help Reduce Global Warming EPSRC Grant Win for WPU and RJKT Groups 3 Organic Seminar Roadside Air Quality Targets May be Met Ahead of 4-5 Speaker: Dr Matt Powner, University College London Schedule Date: Wednesday 10 January Time: 1pm—2pm Journal Honour for Professor John Goodby 5 Location: B/B/006 Chemistry Student Wins Intern of the Year 6 LGBT STEMinar 2018 Erasmus Exchange to Share Good Lab Safety Practice 7 Date: Friday 12 January James Clark Appointed to French National Research Time: 9am—6pm Funding Agency Scientific Committee Location: National STEM Learning Centre EPSRC Dial-a-Molecule 3D Meeting in York 8-9 Physical Seminar Bring it On! 10 Speaker: Dr Patricia Hunt, Imperial College Date: Wednesday 17 January New Starters Time: 1pm—2pm Photocatalyst Library Freely Available in York 11 Location: C/B/101 Chemistry Jon Agirre at Royal Society’s Fellows’ Induction Day 12-13 Research Seminar Speakers: Dr Sarah Moller & Dr Kirsty High Bishopthorpe Runners vs Cancer 13 Date: Friday 26 January TechYork 14 Time: 1pm—2.30pm Location: C/B/102 A Term's Work for the Teaching Labs Instruments 15 Case Studies Wanted 16 Green Chemistry Seminar Speakers: Dr Robin White, Fraunhofer ISE Last Call for Registration for the LGBT STEMinar 2018 Date: Friday 26 January Do You Need to Be a ‘Genius’ to Succeed in 17 Time: 3pm—4pm Academia? Location: C/F/106 Research and Diversity Seminar 18 Equality and Diversity Lunchtime Forum 19 Collection for St. -

View PDF Version
Cutting-edge research for a greener sustainable future www.rsc.org/greenchem Volume 15 | Number 3 | March 2013 | Pages 537–848 ISSN 1463-9262 CRITICAL REVIEW Tom Welton et al. Deconstruction of lignocellulosic biomass with ionic liquids 1463-9262(2013)15:3;1-O Green Chemistry CRITICAL REVIEW View Article Online View Journal | View Issue Deconstruction of lignocellulosic biomass with Cite this: Green Chem., 2013, 15, 550 ionic liquids Agnieszka Brandt,a John Gräsvik,b Jason P. Halletta and Tom Welton*a This paper reviews the application of ionic liquids to the deconstruction and fractionation of lignocellulo- sic biomass, in a process step that is commonly called pretreatment. It is divided into four parts: the first gives background information on lignocellulosic biomass and ionic liquids; the second focuses on the Received 28th August 2012, solubility of lignocellulosic biomass (and the individual biopolymers within it) in ionic liquids; the third Accepted 18th December 2012 emphasises the deconstruction effects brought about by the use of ionic liquids as a solvent; the fourth DOI: 10.1039/c2gc36364j part deals with practical considerations regarding the design of ionic liquid based deconstruction www.rsc.org/greenchem processes. 1. Introduction interest in renewable technologies to replace fossil sources of carbon. One of these technologies is the conversion of During the twentieth century, we came to rely on fossilised biomass to fuels and chemicals in the so-called ‘Integrated organic matter such as coal, gas and oil for the generation of Biorefinery’.1 energy and the production of chemical products. It is now Currently, biofuels are the most significant biomass derived clear that the carbon dioxide produced during combustion of chemicals and are made from edible components of food fossil resources is causing significant climate change. -

Joliot-Curie Conference Programme
16 September 2014 Day 1 – chaired by Eleanor Campbell: 09:30 Registration and arrival refreshments 10:00 Welcome and history of the conference from Hélène Langevin-Joliot 10:30 Mentoring programmes and career breaks Talks on their personal experiences and professional expertise will be given by Rachel Tobbell and Alison Rodger, University of Warwick. This will be followed by a panel discussion with questions from the audience. 11:45 Tea break 12:00 Getting published and generating a public profile Emma Stoye, Chemistry World, and Jane Hordern, RSC Publishing, will talk about getting published and generating a public profile. This will be followed by a panel discussion with questions from the audience. 13:15 Lunch 14:15-17:30 Careers workshop run by Julie Franklin: Introduction to Career Management Career paths: your 50-year career Identifying opportunities: adverts, agencies, networking and social media 16:00 Tea break 16:30 CVs, personal statements, covering letters Interviews and mentoring – asking for feedback and using it 17:30 Closing remarks and questions 18:00 Pre-dinner drinks 19:00 Conference dinner with after-dinner speaker Hosted by Robert Parker, CEO of the Royal Society of Chemistry 17 September 2014 Day 2 – chaired by Carole Morrison 09:00 Registration and arrival refreshments 09:30 Welcome from the Chair 09:45 Diversity and inclusion: unconscious bias Tinu Cornish, Different with a Difference, will lead an interactive session on identifying, understanding and overcoming unconscious bias. 10:45 Tea Break 11:00 Diversity and inclusion: instigating cultural change and Athena SWAN Talks on their personal experiences and professional expertise will be given by Geetha Srinivasan, L'Oréal-UNESCO for Women in Science Fellow, Katriona Methven, Director of Scientific and Regulatory Affairs at L'Oréal and Tom Welton, Head of Chemistry at Imperial College London This will be followed by a panel discussion with questions from the audience. -

Fractionation of Lignocellulosic Biomass with the Ionic Liquid 1- Butylimidazolium Hydrogen Sulfate
CREATED USING THE RSC ARTICLE TEMPLATE (VER. 3.1) - SEE WWW.RSC.ORG/ELECTRONICFILES FOR DETAILS ARTICLE TYPE www.rsc.org/xxxxxx | XXXXXXXX Fractionation of lignocellulosic biomass with the ionic liquid 1- butylimidazolium hydrogen sulfate Pedro Verdía, a Agnieszka Brandt,b Jason P. Hallett,b Michael J. Rayc and Tom Welton*b Received (in XXX, XXX) XthXXXXXXXXX 200X, Accepted Xth XXXXXXXXX 200X 5 First published on the web Xth XXXXXXXXX 200X FDOI: 10.1039/b000000x The application of the protic ionic liquid 1-butylimidazolium hydrogen sulfate in the deconstruction (aka pretreatment) and fractionation of lignocellulosic biomass has been investigated. A cellulose rich pulp and a lignin fraction were produced. The pulp was subjected to 10 enzymatic saccharification which allowed recovery of up to 90% of the glucan as fermentable glucose. The influence of the solution acidity on the deconstruction of Miscanthus giganteus was examined by varying the 1-butylimidazole to sulfuric acid ratio. Increased acidity led to shorter preteatment times and resulted in reduced hemicellulose content in the pulp. Addition of water to the ionic liquid resulted in enhanced saccharification yields. The ability to tune acidity through the 15 use of protic ionic liquids offers a significant advantage in flexibility over dialkylimidazolium analogues. Introduction and have attracted a great deal of interest.4 An increasing number of studies have sought to apply these liquids to the Wide-spread use of petroleum as a feedstock for deconstruction of lignocellulosic biomass.5 Potential transportation fuels and chemicals during the last century has advantages over other methods are the ability of certain ionic 20 provided low-cost energy and materials but also contributed to 55 liquids to dissolve/decrystallise cellulose (Dissolution a historic high in atmospheric carbon dioxide concentrations. -

Diapositiva 1
BIOCATALYSIS FOR THE TREATMENT OF LIGNOCELLULOSIC BIOMASSES U MBERTO CANCELLI ([email protected]) Dept. of Life Sciences - University of Modena and Reggio Emilia, Italy Research Doctorate School in Agri-Food Sciences, Technologies and Bio-Technologies Tutors: Prof. Andrea Antonelli and Prof. Francesca Masino State of the art The lignocellulosic biomass of the grape stalks is principally composed by three biopolymers whole cellulose, hemicellulose and lignin (Figure 1). These three polymers are organized in a complex structure where they are linked by a dense network of ethereal and hydrogen bonds with hemicellulose and lignin which cover cellulose. For this reason this biopolymer is trapped and protected within this structure and it is very difficult to break it into the monosaccharides. Figure 2 Figure 1 Thus it is necessary to break and separate these three biopolymers to obtain the phenolic aromatic moieties from lignin and monosaccharides from hemicellulose and cellulose. The approach of biocatalysis with lignocellulotyc enzymes based on the first use of laccase and then hemicellulase and cellulase represents a valid alternative to the chemical methods of hydrolysis with acids and bases and it allows to obtain the same products, but in a cleaner way (Figure 2). The laccase enzyme, in the pretreatment phase, causes the break of lignin structure to yield aromatic phenolic compounds some with potential industrial interest as buiding blocks for the fine chemistry (e.g vanillin). Hemicellulase and cellulase allow to obtain sugars as glucose and xylose from cellulose and hemicellulose for microbial fermentations, biofuels and biopolymers. Materials & Methods The biocatalytic activity on the lignocellulosic biomass of the grape stalks was made with the enzymes laccase, hemicellulase and cellulase during 24 and 48 hours with 100, 200 Units of enzymatic activity, respectively. -

Royal Society of Chemistry Financial Statements and Trustees' Report
Royal Society of Chemistry Financial Statements and Trustees’ Report 2015 01 Contents We are the world’s Welcome from the President 1 leading chemistry Objectives and strategy 2 community and our mission is to advance Achievements and performance 3 excellence in the Plans for the future 14 chemical sciences. Benevolent Fund 15 Financial review 17 Structure, governance and management 21 Subsidiary companies 23 Reference and administrative details 24 Auditors, bankers and other professional advisers 24 Royal Society of Chemistry Council 25 Responsibilities of the Trustees 26 Independent auditors’ report 27 Consolidated statement of financial activities for year ended 31 December 2015 28 Consolidated balance sheet as at 31 December 2015 29 Royal Society of Chemistry balance sheet as at 31 December 2015 30 Consolidated and charity statement of cash flows for year ended 31 December 2015 31 Notes to the financial statements 32 Welcome from the President I’ve been a member of the Royal Society of Chemistry since Of course, science is international and to solve global I was an undergraduate at the University of Southampton. challenges we need to work together across borders. I’m immensely proud of our organisation and of being a It has been an honour to travel the world during my chemist. presidency, from the United States to Brazil and India, to strengthen links with other centres of chemistry. Last year The chemical and pharmaceutical industry alone is the UK’s we signed a partnership with the British Council, which will largest manufacturing exporter, with exports of nearly £50 help us bring UK chemists together with colleagues through billion each year*. -

9Th Australasian Symposium on Ionic Liquids
9th Australasian Symposium on Ionic Liquids Abstract Booklet 1st-2nd December 2020 Tuesday 1 December - Day 1 Sessions Sydney time Perth time Auckland time Speaker Title 9:30 - 10:00 am 6:30 - 7:00 am 11:30 - 12:00 pm A/Prof Ekaterina (Katya) Pas Welcome - Panel #1-1 Prof Tom Welton, Imperial College, Connecting Ion Structure and Flexibility with the Transport 10:00 - 10:30 am 7:00 - 7:30 am 12:00 - 12:30 pm London, UK Properties of Ionic Liquids - Panel #1-1 Electrochemical Sensor Development for Explosives Detection 10:30 - 10:45 am 7:30 - 7:45 am 12:30 - 12:45 pm Catherine Hay, Curtin University Using Ionic Liquids - Panel #1-1 Reconsidering Intermolecular Interactions in Surface Active Jhonatan Soto Puelles, Deakin 10:45 - 11:00 am 7:45 - 8:00 am 12:45 - 13:00 pm Ionic Liquids as Steel Corrosion Inhibitors – A Modelling Study University - Panel #1-1 11:00 - 11:30 am 8:00 - 9:30 am 13:00 - 13:30 pm Coffee break Designing Novel Solid State Electrolytes based on Karolina Biernacka, Deakin 11:30 - 11:45 am 8:30 - 8:45 am 13:30 - 13:45 pm Hexamethylguanidinium Organic Ionic Plastic Crystal for University Sodium Batteries - Panel #1-2 Quantitative Determination of Protein Solubility in Ionic 11:45 - 12:00 pm 8:45 - 9:00 am 13:45 - 14:00 pm Stuart Brown, RMIT University Liquids - Panel #1-2 Dr. Karolina Matuszek, Monash 12:00 - 12:15 pm 9:00 - 9:15 am 14:00 - 14:15 pm Thermal Energy Storage in Ionic Liquids - Panel #1-2 University Dr. -

Enhancing the Stability of Ionic Liquid Media for Cellulose Processing: Acetal Protection Cite This: Green Chem., 2016, 18, 3758 Or Carbene Suppression?†
Green Chemistry View Article Online PAPER View Journal | View Issue Enhancing the stability of ionic liquid media for cellulose processing: acetal protection Cite this: Green Chem., 2016, 18, 3758 or carbene suppression?† Matthew T. Clough,a,b Jeraime A. Griffith,a Olga Kuzminaa and Tom Welton*a Although excellent candidate solvents for cellulose, capable of dissolving ≥20 wt% of the carbohydrate for electrospinning processes, dialkylimidazolium carboxylate ionic liquids undergo undesirable side reac- tions with the reducing end of saccharides, terminating in an equilibrium concentration of a 2-(hydroxy- methyl)-substituted imidazolium ‘adduct’. The addition of small molar quantities of a benign, non-toxic and inexpensive co-solvent, e.g. glycerol, reduces the rate of adduct accumulation, thereby enhancing the long-term thermal stability and recyclability of the expensive ionic liquid component. NMR, UV-vis and mass spectrometry experiments reveal that the improved stability is likely attributable to suppression of the transient dialkylimidazol-2-ylidene carbene, via hydrogen-donation by the protic co-solvent, rather Creative Commons Attribution 3.0 Unported Licence. than by cyclic acetal protection of the carbohydrate. The incorporation of (up to) 10 wt% of glycerol into Received 5th January 2016, the solvent mixture does not exacerbate the rate of cellulose depolymerisation compared to in the neat Accepted 4th April 2016 ionic liquid, and high solubility of cellulose is maintained. Furthermore, a colourimetric comparison of the DOI: 10.1039/c6gc00027d recovered solvents, following cellulose re-precipitation, demonstrates that glycerol does not increase the www.rsc.org/greenchem concentration of contaminant reducing sugars in the organic electrolyte. This article is licensed under a Introduction lose fibres by initially dissolving crude cellulose in an IL, and then ‘electrospinning’ the viscous solution into an anti-solvent – In view of the rapidly depleting planetary fossil fuel reserves, pool (e.g. -
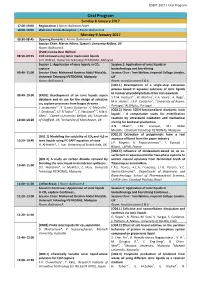
Oral Program
ILSEPT 2017 | Oral Program Oral Program Sunday 8 January 2017 17:00-19:00 Registration | Room: Ballroom Foyer 18:00-19:00 Welcome Drinks Reception | Room: Ballroom B Monday 9 January 2017 08:30-08:45 Opening Remarks | Room: Ballroom A Session Chair: Martin Atkins, Queen's University Belfast, UK Room: Ballroom A [PL01] Cecilia Devi Wilfred 08:50–09:35 CO2 removal using tailor made ionic liquids C.D. Wilfred, Universiti Teknologi PETRONAS, Malaysia Session 1: Application of ionic liquids in CO2 Session 2: Application of ionic liquids in capture biotechnology and biorefining 09:40–11:00 Session Chair: Mohamed Ibrahim Abdul Mutalib, Session Chair: Tom Welton, Imperial College London, Universiti Teknologi PETRONAS, Malaysia UK Room: Ballroom A Room: Function room 5 & 6 [O02.1] Development of a single-step extraction process based in aqueous solutions of ionic liquids to recover phycobiliproteins from red seaweeds 09:40–10:00 [KN01] Development of an ionic liquids aspen 1 1 1 2 S.P.M. Ventura* , M. Martins , F.A. Vieira , A. Rego , database and its use for the design of selective 2 1 1 M.H. Abreu , J.A.P. Coutinho , University of Aveiro, co capture processes from biogas streams 2 2 Portugal, ALGAplus, Portugal J. Jacquemin*1, P. García-Gutierrez2, C. McCrellis2, 2 2 ,3 2 ,3 [O02.2] Novel SO3H-functionalized dicationic ionic I. Dimitriou , S.F.R Taylor , C. Hardacre , R.W.K. 2 1 2 liquids - A comparative study for esterification Allen , Queen’s University Belfast, UK, University 3 reaction by ultrasound cavitation and mechanical 10:00–10:20 of Sheffield, UK, University of Manchester, UK stirring for biodiesel production A.N.