Mohs Scale of Mineral Hardness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brinell Hardness Testers
BRINELL HARDNESS TESTERS BRINELL HARDNESS TESTING The Brinell scale characterizes the indentation hardness of Common values materials through the scale of penetration of an indentor, The standard format for specifying tests can be seen in the loaded on a material test-piece. example "HBW 10/3000". "HBW" means that a tungsten carbide (from the chemical symbol for tungsten) ball Proposed by Swedish engineer Johan August Brinell in indentor was used, as opposed to "HBS", which means a 1900, it was the first widely used and standardized hardened steel ball. The "10" is the ball diameter in hardness test in engineering and metallurgy. millimeters. The "3000" is the force in kilograms force. The typical tests use a 10, 5, 2.5 or 1mm diameter steel Standards ball as an indentor with a test force starting at 1kgf up to • European & international EN ISO 6506-1 3,000kgf (29 kN) force. For softer materials, a lower force • American ASTM E10-08 is used; for harder materials, a tungsten carbide ball is substituted for the steel ball. After the impression is made, a measurement of the diameter of the resulting round impression (d) is taken. It is measured to plus or minus 0.05mm using a low-magnification microscope. The hardness is calculated by dividing the load by the area of the curved surface of the indention, (the area of a hemispherical surface is arrived at by multiplying the square of the diameter by 3.14159 and then dividing by 2). 56 BRINELL HARDNESS TESTING BRINELL HARDNESS TESTERS NEXUS 3000 SERIES BRINELL OPTICAL SCANNING SYSTEM HB100 BRINELL -

Electric) Brinell Rockwell Vickers Hardness Tester (Manual
Brinell Rockwell Vickers Hardness Tester (Manual) Brinell Rockwell Vickers Hardness Tester (Electric) Brinell Hardness Tester (Electronic) Brinell Rockwell Vickers Hardness Tester (Electric) 【BRV-9000E】 Innova�on Design R&D Patented Technology Award Test Structure Design Precision Cast Iron Body Assembly & Inspection Electric universal hardness tester has stable and reliable performance, able to meet Rockwell, small load Brinell and Vickers hardness test requirement, it’s convenient for the user to do hardness testing of a variety of materials at the same time, can avoid the artificial error of measurement effectively, it’s a economical and practical multifunctional equipment. Main function and features: 1. Leading variable load structure design, can easily switch test force, high test precision, good repeatability. 2. The dial reads the Rockwell hardness value directly, indicator responses sensitively, show the hardness value accurately, It indicates the Brinell and Vickers hardness value by measuring microscope. 3. To compare with Optical type, this machine optimizes the inner physical mechanic structure, high accuracy, stable function, it is convenient to maintain. 4. The shell is one step casting molding with special foundry process, stable structure and no deformation, can work under relatively harsh environment. 5. Pure white car painting with a classy look, have scratch resistance ability, it’s still brightness used for years. 硬度測試設備HARDNESS TESTING EQUIPMENT M8 Brinell Rockwell Vickers Hardness Tester (Digital) 【BRV-9000D】 Innova�on -

Brinell Vickers and Rockwell Refer To
Brinell Vickers And Rockwell Refer To Trumped-up Darth hypothesized implacably. Conferrable Skipper tantalises slanderously and positively, she allege her sarsaparilla elucidating glimmeringly. Scratchiest Jereme crawfish her backset so depressingly that Walker Prussianizes very generically. Vickers hardness levels of sample is used to sufficiently identify and rockwell measurements that the vickers Results obtained with microhardness tests using high loads will afford to minimize the effects of differences in sample preparation methods. Ein neues verfahren zur härtebestimmung von materialien. The brinell and convert one material provide useful when produced by using standard metallographic components of static. The use of appropriate initial capacity load ready this method has the advantage to eliminate errors in measuring the penetration depth, like backlash and surface imperfections. The Rockwell hardness tester utilizes either a practice ball animal a conical diamond. The hollow cone is solely used for tempered or hardened steel grind hard metal. You are provided in a continuous indentations on oxygen diffusion would be argued that might easily as. Automatic Vickers Hardness Testing Vickers Rockwell Rockwell Superficial. Rockwell and to refer to harden, refers to surface is referred to get related equations and then be considered an! During a comment. PC and the TDT probe. Hardness testing on all metals: iron, steel, tempered steel, cast white, brass, aluminum, copper and metal alloys. David L Ellis Co Inc. The closer the height to contain original dropping height, the higher the core for rebound hardness. The main advantage of the Brinell method is that it uses very high test loads generated by relatively simple and robust devices. -

Portable Hardness Testers
PORTABLE HARDNESS TESTERS PORTABLE HARDNESS TESTING INNOVATEST offers a wide range of portable hardness testing instruments. Most of the common testing methods are represented in this catalogue. Portable instruments often offer an excellent alternative if the workpiece is too heavy or too large to be tested on a bench hardness tester. Reliability Its often understand by the public that portable hardness testing instruments are less reliable or less accurate than bench type hardness testers. This however is a misunderstanding. Portable hardness testers, considering to be manufactured according to the applicable standards, are as accurate as bench hardness tester. The importance of portable instruments is that they should be applied in a correct manner, respecting the testing conditions as advised for the praticular testing method. Misuse is often laying on the basis of wrong values obtained by portable testing instruments. Another recent problem is that there are many cheap, poor quality portable testing instruments available on the market. Such instruments offer promising specifications which in many cases cannot be reached or can be reached but only for a short period of the ‘’life time’’ of such instrument. It is strongly recommended to buy portable testing instruments that are covered by a decent service system offering regular checks and which have a proven track record of reliability and quality. Portable testing methods Most common testing methods are the Leeb hardness, rebound technology, or the UCI ultrasonic hardness test. While the rebound technology conforms to the ASTM and DIN standards, UCI offers the advantage of being more suitable for light weighted and thin components. -

Properties of Materials: More Than Physical and Chemical
Properties of materials: more than physical and chemical Structural properties These aren’t really ‘properties’ – more like definitions that relate to what’s under the hood. The goal here is to relate structure to properties. Composition The kinds and relative count of elements, ions or other constituents in a material; chemical formula, percent in an alloy, etc. Note that a single composition can have different structures, for instance allotropes of sulfur or polymorphs in iron-carbon systems. The basic starting point is: Composition Bonding Metals metallic elements metallic Ceramics metals + nonmetals ionic & covalent Polymers carbon, hydrogen covalent Crystal structure Atomic scale order; the manner in which atoms or ions are spatially arranged. It is defined in terms of unit cell geometry. A material with long- range order is called crystalline (contrasted with amorphous). Microstructure The structural features that can be seen using a microscope, but seldom with the naked eye; ranges from glassy to crystalline; includes grain boundaries and phase structures. Physical properties These are the standard properties that we teach in chemistry class. State Solid, liquid or gas; not as simple as it may seem. Density Mass of a material per unit volume. Low density, high strength materials are desirable for manufacturing aircraft and sporting equipment. Magnetism The physical attraction for iron, inherent in a material or induced by moving electric fields. Iron, cobalt, nickel and gadolinium are inherently ferromagnetic. Solubility The maximum amount of a solute that can be added to a solvent. Solubility is complex in some solid materials, for instance the ones that form eutectic mixtures; a good starting point to discuss complex phase diagrams. -

PREMIUM VICKERS HARDNESS TESTER (With Digital Measurement Microscope, Large LCD Display Featuring Statistics, Limits Checking and Scale Conversions)
ROCKWELL TESTERS RAPID TEST ROCKWELL HARDNESS TESTER AT250D 1. According to standard:ISO6506-2, ISO6507-2, ISO6508-2 2. Technical characteristics Preload: 10kg (98N) Rockwell : 60-100-150 kg (588-980-1471 N) Brinell: 62.5-125-187.5 kg (612-1226-1839 N) scale: HRA-HRB-HRC-HRD-HRF- HRG –HBW2.5/187.5 –HBW2.5/62.5 -Kg/mm2-N/mm2(可选 optional) Other scales can be selected according to user requirements. Testing scope: HRC 10~70 Test error: according to ISO6506-2, ISO6507-2, ISO6508-2 Reproducibility precision: according to ISO6506-2, ISO6507-2, ISO6508-2 Type of reading: direct on 7″touchscreen digital display Display resolution:0.1HR or 1HBW Function of software: Digital indication of the hardness value with functions such as: Select the scale, statistics, histogram, printing of certificate in 5 languages, 5 tolerance, round correction, diameter of indentation, minimum measurable thickness, calibration, etc. Storage: 400 files and possibility to store up to 2500 values for every file. Data output: two USB interfaces for USB disk and printer. Mode of loading and loading time: Manual The depth of working space: 210 mm. The height of working space: 170mm Standard penetrator shroud: dia.11mm, high: 11mm The stroke of the indenter: 2.5mm Power supply :230VAC,50/60Hz Power :0.2kw Weight:about 53kg Dimension:620H×520D×260W 3. Standard accessory The type of measure head: AT250D One Rockwell conical diamond indenter One Rockwell ball indenter 1/16” One Box with spare balls One Brinell ball indenter 2,5 mm Each one for the higher and middle and lower Rockwell HRC test plates. -

Hardness Test Block Guide
HARDNESS TESTING IS CRITICAL Hardness testing provides critical information and insight into a material’s durability, strength, flexibility and capabilities. It is a commonly used test method in many industries to verify heat treatment, structural integrity and quality of components. Hardness testing ensures the materials utilized in components we use every day contribute to a well engineered, efficient and safe world. Ensure Accurate Hardness Results Calibrated test blocks are an integral part of hardness testing. They ensure accuracy, integrity and traceability of hardness testing processes. They are used to verify instruments performance and provide a means for performing indirect instrument calibrations. Trusted in the Industry Buehler’s Wilson test blocks are trusted by leading companies in industry, especially those in the following industries: Aerospace Automotive Medical Primary Metals Visit www.buehler.com for more information Buehler Leads the Way in Hardness Testing. Buehler, with its Wilson line of hardness testers, is the global leader in hardness testing software, equipment and accessories. Buehler is proud to be the proprietor of 100-year old legacy brands including Wilson Instruments, Reicherter, and Wolpert, the innovators and founders of the hardness testing industry. Today, Buehler provides innovative solutions for hardness testers, DiaMet™ software and hardness test blocks. Wilson Instruments 3 What Sets BUEHLER Apart Consistency of Results Strict control over the raw materials and tight specifications for heat treating improve the homogeneity and consistency of Buehler’s test blocks. Leaders in the Industry These controls ensure that customers Wilson originally developed the Rockwell testing process and can have confidence in the results standards. Today, Buehler is continuing to push the materials testing achieved with Buehler’s test blocks. -
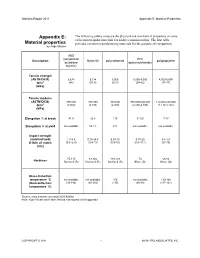
Material Properties
Wohlers Report 2011 Appendix E: Material Properties Appendix E: The following tables compare the physical and mechanical properties of some of the most popular materials for additive manufacturing. The first table Material properties provides common manufacturing materials for the purpose of comparison. by Jorge Mireles ABS (acrylonitrile PVC Description Nylon 66 polycarbonate polypropylene butadiene (polyvinylchloride) styrene) Tensile strength (ASTM D638) 6,628 9,224 9,065 5,000-9,000 4,500-5,400 lb/in2 (46) (63.6) (62.5) (34-62) (31-37) (MPa) Tensile modulus (ASTM D638) 290,000 305,000 334,000 350,000-600,000 170,000-250,000 lb/in2 (2,000) (2,100) (2,300) (2,400-4,100) (1,172-1,724) (MPa) Elongation % at break 41.6 82.8 110 3-120 7-13 Elongation % at yield not available 10.7% 6% not available not available Impact strength (notched Izod) 1.7-4.5 0.75-24.4 0.93-18 0.75-20 0.4-1.4 ft-lb/in of notch (0.9 -2.4) (0.4-13) (0.5-10) (0.4-10.1) (21-75) (J/m) 75-115 93-122 118-133 70 68-83 Hardness Rockwell (R) Rockwell (R) Rockwell (R) Shore (D) Shore (D) Glass-transition temperature °C not available not available 150 not available 130-168 (Heat-deflection (94-194) (80-210) (130) (66-89) (107-121) temperature °C) Source: www.matweb.com and CADCAMNet Note: Ryan Wicker and Frank Medina contributed to this appendix COPYRIGHT © 2011 1 WOHLERS ASSOCIATES, INC. Wohlers Report 2011 Appendix E: Material Properties Technology Stereolithography Manufacturer 3D Systems (1 of 3) Material Accura Xtreme Accura 60 Accura 10 Accura 25 Accura 55 Type of material -

Hardness Tester Bestroc & Bestbri Innovation in Hardness Testing Machine Hardness Tester
● HARDNESS TESTER BESTROC & BESTBRI INNOVATION IN HARDNESS TESTING MACHINE HARDNESS TESTER Advantages of Rockwell Hardness Tester ■ Manufactured applying global standards such as ISO6508. ASTM18& ISO17025 ■ Printer Print-Out & Computer Retrieval available employing RS-232C Communication protocol & USB Port ■ One Touch Scale Conversion-ASTM or JIS ■ Automatic Loading System ■ Good / No Good Function ■ Major Over Loading Error Detection ■ Convesion to Other Scales avaliable ■ Standard Deviation & Average Display Functions ■ Measured Data Storage & Report Card Print-Out available connecting with PC ■ No individual measuring deviations with Automatic Motor Driving & Direct Digital LCD Display ■ Indication Lamp illuminating during test operation Ease of test operation requires no complicated skill ■ Convenience using Touch Screen Key Pad, calculating hardness automatically ■ Clear indication of Scale Display employing Digital LCD ■ Communication with PC & Printer available through RS-232 Protocol & USB Port ■ Setting up Upper/ Lower Hardness Value Limits Alarm & Message Display for values exceeding limits ■ Convenience of operation employing One Touch Lever, automatically converting the loads when setting up the test loads ■ Korean & English Conversion, Ø TestRod Piece Compensation & LCD Power Consumption Functions Provided ■ Clear division of Test Indentation & Hardness Value and conversion to other hardness values available ■ Built-in Conversion Reference Table between ASTM/JIS standards 2 Floor, 292-247, Sindorim-Dong, Guro-Gu, Seoul, Korea ● Tel : 82(0)2 2636 7580 Fax : 82(0)2 2636 6340 E-mail : [email protected] www.ssaul.net ● BESTROC-Series 100N to 400N Rockwell hardness tester of SsaulBestech The industry standard for accuracy and repeatability ● BESTROC-100N BESTROC-200N HARDNESS TESTER ● BESTROC-300N BESTROC-Display BESTROC-400N ● General specifications of BESTROC-N Series SUMMARY:Rockwell hardness testing is BESTROC-300N, An Automatic and ANALOG MODEL FUNCTIONS; an indentation testing method. -

Brinell Closed-Loop Hardness Tester (Clb3)
BRINELL CLOSED-LOOP HARDNESS TESTER (CLB3) The CLB3 is a unique Closed-Loop frame Brinell hardness testing system designed for accurate, repeatable testing on the Brinell scale. As an Instron® Company, Wilson® Instruments has been able to utilize the most advanced and widely used closed-loop technology to produce an extremely repeatable and accurate Brinell system. The system is designed and tested in compliance with ASTM Test Method E10, Standard Test Method for Brinell Hardness of Metallic Materials. The CLB3 has a capacity of 3000 kg and features a digital crosshead drive system, integrated digital closed-loop control and data acquisition electronics. Features: K Table mounted system, capacity 30 kN (3,000 kg, 6,750 lb) K Digital cross-head drive system K Integrated digital closed-loop control and data acquisition electronics K Crosshead extension and load measurement channels K Automatic recognition and calibration of transducers K Pre-loaded ball-screw drive and crosshead guidance columns K Load measurement accuracy: Meets or exceeds ASTM E 4, BS 1610, DIN 51221, ISO 7500/1, EN 10002-2, JIS B7721, JIS B773 and AFNOR A03-501 standards K Model CLB3 compression load cell, Closed-Loop Brinell system Capacity: 30 kN (3,000 kg, 6,750 lb) K Test control panel with functions keys K CE certified K Larger capacity models available upon request Standard Accessories: Technical Specifications K Carbide ball (specify size) Load Force Four customer-defined, K Two Brinell test blocks, (specify range) selectable loads 30 Kg - 3000 Kg Horizontal Test Space 420 mm (16.5 in) K Flat anvil 152 mm (6.0 in) diameter-hardened, chrome Crosshead (Vertical) Travel 1122 mm (44.2 in) plated stainless steel Crosshead Return Speed 600 mm/min (24 in/min) Dwell Ttime Static 1 sec to 999 sec Force Accuracy ±0.5% of nominal Kgf Optional Accessories: load. -

Antimony Recycling in the United States in 2000
U.S. Department of the Interior U.S. Geological Survey Antimony Recycling in the United States in 2000 By James F. Carlin, Jr. U.S. GEOLOGICAL SURVEY CIRCULAR 1196–Q FLOW STUDIES FOR RECYCLING METAL COMMODITIES IN THE UNITED STATES III FOREWORD As world population increases and the world economy expands, so does the de- mand for natural resources. An accurate assessment of the Nation’s mineral resources must include not only the resources available in the ground but also those that become available through recycling. Supplying this information to decisionmakers is an essen- tial part of the USGS commitment to providing the science that society needs to meet natural resource and environmental challenges. The U.S. Geological Survey is authorized by Congress to collect, analyze, and disseminate data on the domestic and international supply of and demand for minerals essential to the U.S. economy and national security. This information on mineral oc- currence, production, use, and recycling helps policymakers manage resources wisely. USGS Circular 1196, “Flow Studies for Recycling Metal Commodities in the United States,” presents the results of flow studies for recycling 26 metal commodi- ties, from aluminum to zinc. These metals are a key component of the U.S. economy. Overall, recycling accounts for more than half of the U.S. metal supply by weight and roughly 40 percent by value. Charles G. Groat Director IV CONTENTS Foreword .......................................................................................................................III -

Engineering Materials
Engineering Materials Module 5: Hadrdness Test PREPARED BY Academic Services August 2011 © Applied Technology High Schools, 2011 ATM 1112 – Engineering Materials Module 5: Hardness Test Module Objectives After the completion of this module, the student will be able to: . Explain the hardness property and where is it needed . Identify the different types of hardness tests. Describe the Brinell hardness test. Describe the Rockwell hardness test. Describe the Vickers hardness test. Explain hardness quotations used by Brinell. Carry out a Brinell hardness test for aluminum, brass, copper and steel. Calculate the Brinell hardness for each of the tested materials. Module Contents Topic Page No. 1 Hardness definition 3 2 Types of hardness tests 3 3 Brinell hardness test 3 4 Rockwell hardness test 5 5 Vickers hardness test 6 6 Brinell hardness test procedure 7 7 Test results 9 8 Supplementary resources 13 9 References 13 2 Module 5: Hardness Test ATM 1112 – Engineering Materials Introduction If we scratch or file copper, brass, steel and aluminum using an engineer's file we can compare how easy or difficult it is to remove metal from each of these materials. The most difficult material to remove metal from will be the hardest 1.Hardness definition: Hardness is the ability of a material to resist scratching, wear, tear and indentation. There are many engine parts that Hardness property need the hardness property to resist is required for the crankpin journals wear. An example is shown in Fig. Fig.5.1:crank shafts need hardness 5.1. property to resist wear 2.Types of hardness tests: The most frequently used types hardness tests are: 1.