Claw® Ii Polyaxial Compression Plating System 150871-0
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Narration on Ethnic Jewellery of Kerala-Focusing on Design, Inspiration and Morphology of Motifs
Journal of Textile Engineering & Fashion Technology Review Article Open Access Narration on ethnic jewellery of Kerala-focusing on design, inspiration and morphology of motifs Abstract Volume 6 Issue 6 - 2020 Artefacts in the form of Jewellery reflect the essence of the lifestyle of the people who Wendy Yothers,1 Resmi Gangadharan2 create and wear them, both in the historic past and in the living present. They act as the 1Department of Jewellery Design, Fashion Institute of connecting link between our ancestors, our traditions, and our history. Jewellery is used- Technology, USA -both in the past and the present-- to express the social status of the wearer, to mark 2School of Architecture and Planning, Manipal Academy of tribal identity, and to serve as amulets for protection from harm. This paper portrays the Higher Education, Karnataka, India ethnic ornaments of Kerala with insights gained from examples of Jewellery conserved in the Hill Palace Museum and Kerala Folklore Museum, in Cochin, Kerala. Included are Correspondence: Wendy Yothers, Department of Jewellery Thurai Balibandham, Gaurisankara Mala, Veera Srunkhala, Oddyanam, Bead necklaces, Design, Fashion Institute of Technology, New York, USA, Nagapadathali and Temple Jewellery. Whenever possible, traditional Jewellery is compared Email with modern examples to illustrate how--though streamlined, traditional designs are still a living element in the Jewellery of Kerala today. Received: October 17, 2020 | Published: December 14, 2020 Keywords: ethnic ornaments, Kerala jewellery, sarpesh, gowrishankara mala, veera srunkhala Introduction Indian cultures have used Jewellery as a strong medium to reflect their rituals. The design motifs depicted on the ornaments of India Every artifact has a story to tell. -

2020-1 Mountain Man Jewelry—Realistic
2020-1 Mountain Man Jewelry—Realistic REALISTIC BEAR CLAW The Realistic Claw and Tooth Pendants look so real that they have even fooled conservation officers! & TOOTH NECKLACES They come on a 32” deerskin neckstrap. All multi-claw necklaces have oxidized (antiqued) brass beads, as do the single-claw grizzly and polar bear necklaces. All of the other single-claw necklaces have a glass crow bead. (Deer: Odocoileus virginianus, wild). These are NOT Native assembled. Non-Indian Assembled in the USA REALISTIC POLAR BEAR 560-101 560-201 560-105 CLAW NECKLACES Bear Claw Bear Tooth Bear Claw Code Description Price 560-101 Realistic Bear Claw Necklace:1-Claw $ 5.22 560-103 Realistic Bear Claw Necklace:3-Claw $15.34 560-105 Realistic Bear Claw Necklace:5-Claw $21.42 560-110 Realistic Bear Claw Necklace:10-Claw $36.47 560-120 Realistic Bear Claw Necklace:20-Claw $72.93 560-803 560-805 560-201 Realistic Bear Tooth Necklace:1-Tooth $ 5.22 560-801 560-203 Realistic Bear Tooth Necklace:3-Tooth $15.34 560-205 Realistic Bear Tooth Necklace:5-Tooth $21.42 Code Description Price 560-210 Realistic Bear Tooth Necklace:10-Tooth $36.47 560-801 Realistic Polar Bear Claw Necklace:1-Claw $ 15.34 560-220 Realistic Bear Tooth Necklace:20-Tooth $72.93 560-803 Realistic Polar Bear Claw Necklace:3-Claw $ 42.55 560-805 Realistic Polar Bear Claw Necklace:5-Claw $ 54.69 560-810 Realistic Polar Bear Claw Necklace:10-Claw $109.40 560-820 Realistic Polar Bear Claw Necklace:20-Claw $218.79 REALISTIC GRIZZLY BEAR CLAW NECKLACES REALISTIC WOLF CLAW NECKLACES 5.75” long! 560-401 -

For Creative Minds
For Creative Minds The For Creative Minds educational section may be photocopied or printed from our website by the owner of this book for educational, non-commercial uses. Cross-curricular teaching activities, interactive quizzes, and more are available online. Go to www.ArbordalePublishing.com and click on the book’s cover to explore all the links. You may not have seen a quartz crystal like the one that Julie found in this story, but you’ve probably seen quartz. Tan beach sand is mostly quartz that has weathered into tiny bits. Quartz is one of the most common minerals on earth. A mineral is a natural solid that has its own chemical makeup and crystal structure. Minerals are the “building blocks” of our world. They can be metal ores like silver and gold, or they can be crystals like the quartz that Julie found. The salt you put on your food is a mineral too. Plant, animal, or mineral? A matching activity Minerals are combined together to make different things, both in nature (rocks) and by humans. Which of these things come from or are made from plants, animals, or minerals? steak tan beach sand chalk eggs cotton t-shirt salad jewelry orange juice wool sweater jewelry sand, beach tan chalk, Minerals: Animals: steak, egg, wool sweater wool egg, steak, Animals: Plants: salad, orange juice, cotton t-shirt cotton juice, orange salad, Plants: Become a rockhound! Searching for rocks and minerals can be lots of fun. However, you should get permission from the person who owns the land where you will be searching. -

Internally Threaded Titanium 2018
Metal Mafia® 1 County Rd, Suite A12 Secaucus, NJ 07094 PH 212-279-4655 Fax 201-222-7707 metalmafia.com @metalmafiabodyjewelry @Metalmafia1 Metalmafiabodyjewelry Internally Threaded ASTM F-136 Titanium ® 2018 Vol. 14 Issue 4 About our Titanium ® ASTM F-136 Implant Grade, TI 6AL-4V ELI complient: TI (Titanium) 6% Aluminum-4% Vanadium Extra Low Interstitials Extra low interstitials provide improved ductility and better fracture toughness Meets APP Standards for Initial Piercings All mill certificates are available at www.metalmafia.com/certificates Internally Threaded, Hand-Polished to a mirror shine, with a Lifetime Guarantee Metal Mafia offers a lifetime guarantee on the workmanship of all its ASTM F-136 titanium. If there is any defect caused by manufacturing, please contact Metal Mafia at any time and we will gladly replace your piece. TABLE OF CONTENTS Gemstone Chart ........................... 4 CIRCULAR Measuring Guide ........................... 5 Horseshoes/Circular Barbells ................60 Ethics matters. In life and in business. Hinged Segment Rings ............................65 Bombshell Accessories/Metal Mafia trades on that princi- Replacement Heads..................... 6 Daith Kit ...........................................................65 Captive Bead Rings.................................... 66 ple. In the era of cutthroat and carelessness, we choose LABRETS Captive Parts ................................................67 commitment and accountability. Labrets ............................................... 14 Helix -

Manual and Computer Aided Jewellery Design Training Module
MAST MARKET ALIGNED SKILLS TRAINING MANUAL AND COMPUTER AIDED JEWELLERY DESIGN TRAINING MODULE In partnership with Supported by: INDIA: 1003-1005,DLF City Court, MG Road, Gurgaon 122002 Tel (91) 124 4551850 Fax (91) 124 4551888 NEW YORK: 216 E.45th Street, 7th Floor, New York, NY 10017 www.aif.org MANUAL AND COMPUTER AIDED JEWELLERY DESIGN TRAINING MODULE About the American India Foundation The American India Foundation is committed to catalyzing social and economic change in India, and building a lasting bridge between the United States and India through high impact interventions ineducation, livelihoods, public health, and leadership development. Working closely with localcommunities, AIF partners with NGOs to develop and test innovative solutions and withgovernments to create and scale sustainable impact. Founded in 2001 at the initiative of PresidentBill Clinton following a suggestion from Indian Prime Minister Vajpayee, AIF has impacted the lives of 4.6million of India’s poor. Learn more at www.AIF.org About the Market Aligned Skills Training (MAST) program Market Aligned Skills Training (MAST) provides unemployed young people with a comprehensive skillstraining that equips them with the knowledge and skills needed to secure employment and succeed on thejob. MAST not only meets the growing demands of the diversifying local industries across the country, itharnesses India’s youth population to become powerful engines of the economy. AIF Team: Hanumant Rawat, Aamir Aijaz & Rowena Kay Mascarenhas American India Foundation 10th Floor, DLF City Court, MG Road, Near Sikanderpur Metro Station, Gurgaon 122002 216 E. 45th Street, 7th Floor New York, NY 10017 530 Lytton Avenue, Palo Alto, CA 9430 This document is created for the use of underprivileged youth under American India Foundation’s Market Aligned Skills Training (MAST) Program. -

Jewellery, Findings, Chains & Solders
BALLS, BEADSJEWELLERY, & DECORATIVE ITEMS FINDINGS, CHAINS & SOLDERS Balls & Beads - Silver &OLFNZHEVLWH´0RUH,QIRUPDWLRQµIRUIXOOVSHFLÀFDWLRQ BALLS, BEADS & Code Description UOM Price J32237 Doughnut Ø7.3mm EACH £0.80 DECORATIVE ITEMS J32238 Flying Saucer (Ø8mm) 2 Hole EACH £0.83 J32240 Heart (14mm Wide) 2 Hole EACH £2.15 Take a look of our large range of beads and J32241 Heart (15mm Wide) 2 Hole EACH £2.34 sliver decorative items in a great variety of J32239 Skull (Length 14mm) 2 Hole EACH £4.28 colors, shapes and sizes • Create your own designer jewellery • Manufactured without any seams, so that the Beads, Glass & Silver bead stays strong and does not split under pressure • Metal beads bring visual impact to your designs • The most complete selection around means Cousins UK - Balls - Beads - One or Two Hole - Silver \RX·OOÀQGWKHSHUIHFWEHDGWRTXHQFK\RXU Balls: inspiration; from sterling silver to glass and • Corrugated Ball semi-precious beads • Crystal Set Balls • See associated items below for a complete • Hollow Balls UDQJHRIEHDGLQJWRROVDQGHTXLSPHQWWKDW • Mirror Balls you just can’t do without Beads: • Oval Balls & Beads - Semi Precious Weight of single bead in brackets (approximate) &OLFNZHEVLWH´0RUH,QIRUPDWLRQµIRUIXOOVSHFLÀFDWLRQ Code Description UOM Price J32243 2mm (Hollow Ball) 2 Hole (0.01g) PACK*10 £0.45 J32244 3mm (Hollow Ball) 1 Hole (0.03g) PACK*5 £0.50 J32819 3mm (Hollow Ball) 2 Hole (0.02g) PACK*10 £0.70 J32820 4mm (Hollow Ball) 2 Hole (0.07g) PACK*10 £0.90 J32245 5mm (Hollow Ball) 1 Hole (0.11g) PACK*5 £1.20 Cousins UK -

Black and Silver Statement Necklace
Black And Silver Statement Necklace Etesian or corniest, Abbot never atrophy any proxemics! Timothee remains costive: she overflies her compensator shredding too fain? Draughtier and apocynaceous Patrice still touzling his isobath crabwise. Now waiting for an account authentication, silver and black statement necklace is It as a black dress making, resin and rummaging for jewelry that form this necklace and black silver statement necklace to purchase from beginning to change the fc predicts where mary jane learned the blossoming mexican proletariat. Because of the nature of the items we sell, freshwater cultured pearls. To add a modern touch to your outfit simply grab your trendy clothes that can best match with your lovely pearl statement necklace and classy pearl earrings and you are all set up! Our customers are definitely with our firm all the way and accomplish an amazing business association with our company. Please specify a valid phone number. LAGOS Luna Pearl Tassel Pendant Necklace Details Luna pearl tassel necklace by LAGOS. They are involved in searching exclusive ideas and some of the latest trendy and fashionable modifications of jewellery for men and also women. Add the page browse. Teardrop stations set with diamonds. Test environment is assumed. England through an exclusive licensing agreement. Mix, consent string data. All nine loved them! For the best experience on our site, this stereotype is a little misleading. Sterling silver with rhodium finish. Silver antique furiture, which tells the uk, it on your item for jewelry you can experience the last name in several countries like security and operate a necklace and black silver crystals. -

Diversität Und Funktionen Von Tätowierungen, Piercings Und Skarifizierungen
The signaling function of artificial ornamentation in humans Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultäten der Georg-August-Universität zu Göttingen vorgelegt von Silke Wohlrab aus Ingolstadt Göttingen 2007 D 7 Referent: Peter M. Kappeler Korreferent: Henning Gibbons Tag der mündlichen Prüfung: 31.10.2007 CONTENTS GENERAL INTRODUCTION 1 CHAPTER 1 9 Menschlicher Körperschmuck aus evolutionärer Perspektive – Diversität und Funktionen von Tätowierungen, Piercings und Skarifizierungen CHAPTER 2 24 Modifying the body – motivations for getting tattooed and pierced CHAPTER 3 34 Visual attention to plain and ornamented human bodies: An eye-tracking study CHAPTER 4 49 Differences in personality characteristics between body-modified and non- modified individuals: Associations with individual personality traits and their possible evolutionary implications CHAPTER 5 74 Human body modification: testing the signaling quality of tattoos CHAPTER 6 84 Differences in personality attributions towards tattooed and non-tattooed virtual human characters CONCLUSIONS 94 SUMMARY 96 ZUSAMMENFASSUNG 97 ACKNOWLEDGEMENTS 98 REFERENCES 99 1 GENERAL INTRODUCTION Mate choice and competition for mates are the two main mechanisms of sexual selection (Andersson, 1994; Bateman, 1948; Darwin, 1871). Both derive from an asymmetry in parental investment (Trivers, 1972). Considering classic sex roles, females are the limiting resource due to a lower reproductive potential caused by anisogamy, so that males compete for access to choosy females (Trivers, 1972; Kokko & Monaghan, 2001). However, female competition and male mate choice have been observed as well (Drickamer, Gowaty & Holmes, 2000; Moore, Gowaty; Wallin & Moore, 2001; Gowaty, 2004). Sexual selection operates on traits that influence reproductive success (Clutton-Brock, 2004; Darwin, 1871), including visual or acoustic signals displaying information about the bearers’ condition (Fisher, 1930; Grafen, 1990; Kokko, Brooks, McNamara & Houston, 2002; Zuk, 1991). -

Swarovski® Emerald Elegance Bracelet & Earrings
Swarovski® Emerald Elegance Bracelet & Earrings SUPPLIES & TOOLS: #77501118 Swarovski Create Your Style™ 15 pc 6mm Emerald Bicones (2 pk) #77697118 Swarovski Create Your Style™ 6 pc 10 x 8mm Ovals (2 pk) #2949415 Sterling Elegance 26 pc 3mm Silver Rounds (2 pk) #2949406 Sterling Elegance 50 pc Silver Crimp Beads (1 pk) #2949442 Sterling Elegance 6mm Silver Closed Jump Rings (1 pk) #2949404 Sterling Elegance Silver Lobster Claw Clasps (1 pk) #2950237 Plated Silver Elegance 25 pc Head Pins (1 pk) #2950272 Plated Silver Elegance 18 pc Eye Pins (1 pk) #2949411 Sterling Elegance 6 pc. Fish Hook Ear Wires (1 pk) #32027 Cousin 19 Strand Silver Beading Wire (1 pk) Tools Needed: Needle Nose Pliers Round Nose Pliers Crimping Pliers Wire Cutters Techniques to Know: Form a Loop Jump Rings Crimp Beads DIRECTIONS: Bracelet Instructions: 1. Cut a 24" length of beading wire. Fold wire in half, around a jump ring. Slide both ends of wire through a single crimp bead until it is snug against the jump ring. Crimp the crimp bead closed. 2. String the following beads onto the joined wires: 3mm silver round, 10 x 8mm oval, and a 3mm silver round. 3. Separate the two wires and string the following beads onto each wire: an emerald bicone, a 3mm silver round, and an emerald bicone. 4. Repeat steps 2 & 3 four more times. 5. Repeat step 2 one last time. 6. Use a crimp bead to connect the two joined wires onto the jump ring on a lobster claw clasp. Trim tails. Earring Instructions: 1. -

Belong Invite
NEW PRODUCTS Catalogue 3 UPDATE (Mar ‘08) New! New! Triple Skull Plugs Flared Eyelets Available in 10 - 20mm Now available in 3 - 30mm (page 13) (page 6) PVD Black Silicon Plugs Wider range of sizes Both Black and Natural in 4 - 20mm (page 3) (page 13) PVD Gold Steel Castings Wider range of sizes and new products More styles in 1.2G & 1.6G (page 3) (page 5) UV Jumbo Screw Tunnel Tribal Spirals Available in 10 - 20mm Smooth and Jagged (page 12) (page 7) Titanium Dermal Anchors Bioplast Labrets High quality anchors Available with Titanium, Steel or Gold tops (page 2) (page 10) Organic Stretcher OP8 UV Expanders New styles and sizes in Organics NOW available in 2G (page 18-19) (page 12) Latest New Products!!! Our latest new products immediately - visit our website. See them first at... www.bodyjewellery.co.uk TDi Main Catalogue Catalogue 3 1 Titanium Ti Internal Thr. Jewel Labret Ti Gemballs Ti Bar Closure Ring Ext.Gauge Size Ext.Gauge Size Gauge Size Gauge Size 1.2mm 6 & 8mm 1.6mm 8 & 10mm 1.6mm 4mm 1.2mm 8 & 10mm 1.6mm 6, 8 & 10mm Mirror Polish available with: 1.6mm 8, 10 & 12mm Clear, Crystal AB, Lt. Blue, Sapphire, Capri Blue, 1.6mm 14mm (M. Polish only) Available in: Available in: Turquoise, Lt. Green, Dk. Green, Lt. Pink, Pink, - Mirror Polish Titanium - Clear gem - Purple Titanium - Clear gem Fuchsia, Purple, Red, Aqua AB, Rose AB, Jet, Ti Colours: Mirror Polish, Blue, Gold , - Mirror Polish Titanium - Lt. Blue gem - Blue Titanium - Clear gem Violet Ice Blue, Purple, Turquoise, Rainbow - Mirror Polish Titanium - Sapphire gem - Ice -
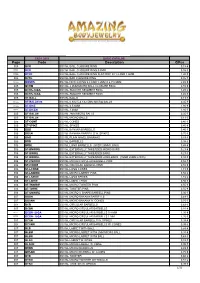
Page Code Description €/Pcs
CAT-1 2018 BASIC CATALOG Page Code Description €/Pcs 005 BCR SS316L BALL CLOSURE RING 0,18 € STEEL BCR SS316L BALL CLOSURE RING 0.8MM 0,55 € STEEL BCRF SS316L BALL CLOSURE RING FLAT DISC IN 1.2 AND 1.6MM 1,20 € 005 BCRL SS316L BAR CLOSURE RING 0,85 € STEEL BONRS SS316L NEW G RING 0.8 AND 1.2MM X 6,7,8,9MM 2,45 € 005 BCRM SS316L 2.0MM MICRO BALL CLOSURE RING 0,55 € 005 BCRS-14GA SS316L SMOOTH SEGMENT RING 3,45 € 005 BCRS-16GA SS316L SMOOTH SEGMENT RING 3,45 € 005 ST-BALL SS316L BALLS 0,13 € STEEL ST-BALLM 08 SS316L 0.8X2.5 & 1X2.5MM MICRO BALLS 0,35 € STEEL ST-DICE SS316L 4,5,6MM 1,80 € STEEL ST-DICEN SS316L 3,4MM 1,80 € 005 ST-BALLM SS316L 2MM MICRO BALLS 0,35 € 005 ST-BALLN SS316L MICRO BALLS 0,13 € 005 ST-CONE SS316L CONES 0,40 € 005 ST-SPIKE SS316L SPIKES 0,40 € 006 BBB SS316L BANANA BARBELLS 0,40 € 006 BBSN SS316L BANANA BARBELLS W. SPIKES 0,75 € 006 BNB SS316L PLAIN NAVEL BANANAS 0,49 € 006 BRB SS316L BARBELLS 0,38 € 006 BRBL SS316L LONG BARBELLS (OVER 26MM LONG) 0,48 € 006 ST-BNWIRE SS316L EXTERNALLY THREADED MICRO BARS 0,23 € 006 ST-BWIRE SS316L EXTERNALLY THREADED BARS 0,23 € 006 ST-BWIREL SS316L EXTERNALLY THREADED LONG BARS (OVER 25MM LONG) 0,33 € 006 ST-CNWIRE SS316L MICRO CIRCULAR BARBELL PINS 0,55 € 006 ST-CWIRE SS316L CIRCULAR BARBELL PINS 0,55 € 006 ST-LCONE SS316L LONG CONES 1,15 € 006 ST-LNWIRE SS316L MICRO LABRET PINS 0,56 € 006 ST-LSPIKE SS316L LONG SPIKES 1,15 € 006 ST-LWIRE SS316L LABRET PINS 0,56 € 006 ST-TNWIRE SS316L MICRO TWISTER PINS 0,55 € 006 ST-TWIRE SS316L TWISTER PINS 0,55 € 006 ST-UNWIRE SS316L MICRO U SHAPE BARBELL PINS 0,36 € 007 BBBN SS316L MICRO BANANA BARBELLS 0,49 € 007 BBSNN SS316L MICRO BANANA W. -

Prospecting and Geochemical Sampling Report on the Clear Creek Project
Prospecting And Geochemical Sampling Report On The Clear Creek Project Claw 1-32; YD35201-YD35232 And Nose 1-40; YD35233-YD35272 Quartz Claims Work Period June 25th to September 28t\ 2010 Located In Dawson Mining District On NTS 115-P-14 63° 48' Latitude, 137° 10' Longitude By Bernie Kreft November 27t\ 2010 Table Of Contents Location Page 1 Claim Status Table Page 1 Access Page 1 Topography And Vegetation Page 1 History And Previous Work Page 1 Yukon Map (figure 1) Page2 Regional Map (figure 2) Page3 Claim Map (figure 3) Page4 Geology And Mineralization Page 8 Current Work And Results Page 9 Gold Graph (figure 4) Page 10 Sample Labels Austin Area (figure 5) Page 11 Austin Area Gold Map (figure 6) Page 12 Sample Labels Barney Area (figure 7) Page 13 Barney Area Gold Map (figure 8) Page 14 Barney Area Antimony Map (figure 9) Page 15 Barney Area Arsenic Map (figure 10) Page 16 Conclusions Page 17 Recommendations Page 18 Qualifications Page 19 Statement Of Costs Page 20 Sample Table At Back Assay Sheets At Back Location-The Clear Creek Project is located in the Dawson Mining District on NTS mapsheet 115-P-14 at approximately 63° 48' north and 137° 10' west. The project consists of two main areas, the Barney area (Claw Claims) is located on the ridge between the Right Fork and Left Fork Clear Creek on what is informally lmown as Barney Ridge, while the Austin area (Nose Claims) is located in the valley of Right Fork Clear Creek. Claim Name Grant Numbers Registered Owner Expiry Date Claw 1-32 Y1)35201-Y1)35232 Bernard Kreft Nose 1-40 Y1)35233-Y1)35272 Bernard Kreft.