Zoologische Verhandelingen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bioecology and Management of Giant African Snail, Achatina Fulica (Bowdich)
INTERNATIONAL JOURNAL OF PLANT PROTECTION e ISSN-0976-6855 | Visit us : www.researchjournal.co.in VOLUME 7 | ISSUE 2 | OCTOBER, 2014 | 476-481 IJPP A REVIEW DOI : 10.15740/HAS/IJPP/7.2/476-481 Bioecology and management of giant African snail, Achatina fulica (Bowdich) BADAL BHATTACHARYYA*1, MRINMOY DAS1, HIMANGSHU MISHRA1, D.J. NATH2 AND SUDHANSU BHAGAWATI1 1Department of Entomology, Assam Agricultural University, JORHAT (ASSAM) INDIA 2Department of Soil Science, Assam Agricultural University, JORHAT (ASSAM) INDIA ARITCLE INFO ABSTRACT Received : 30.06.2014 Giant African snail (Achatina fulica Bowdich) belongs to the Phylum–Mollusca and Class– Accepted : 21.09.2014 Gastropoda. It is known for its destructive nature on cultivated crops wherever it occurs and is one of the world’s largest and most damaging land snail pests. The pest is an East African origin, has spread in recent times by travel and trade to many countries. They now widely KEY WORDS : distributed and no longer limited to their region of origin due to several factors viz., high Bioecology, Management, Giant reproductive capacity, voracious feeding habit, inadequate quarantine management and human African snail, Achatina fulica aided dispersal. A. fulica can cause serious economic damage on different crops and extensive rasping (scrapping), defoliation, slime trials, or ribbon like excrement is signs of infestation. In recent times, severe outbreak of this pest has been noticed due to some desirable agricultural and gardening practices like minimum tillage practices and straw retention techniques which help in survival of snails and make seedlings more susceptible to damage. This review paper aims to enlighten on taxonomy, distribution, extent of damage, morphology, biology, ecology, homing behaviour, seasonal incidence, nature of damage, host plants of A. -

Conchological Differentiation and Genital Anatomy of Nepalese Glessulinae (Gastropoda, Stylommatophora, Subulinidae), with Descriptions of Six New Species
A peer-reviewed open-access journal ZooKeys 675: 129–156Conchological (2017) differentiation and genital anatomy of Nepalese Glessulinae... 129 doi: 10.3897/zookeys.675.13252 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Conchological differentiation and genital anatomy of Nepalese Glessulinae (Gastropoda, Stylommatophora, Subulinidae), with descriptions of six new species Prem B. Budha1,3, Fred Naggs2, Thierry Backeljau1,4 1 University of Antwerp, Evolutionary Ecology Group, Universiteitsplein 1, B-2610, Antwerp, Belgium 2 Na- tural History Museum, Cromwell Road, London, SW7 5BD, UK 3 Central Department of Zoology, Tribhuvan University, Kirtipur, Kathmandu, Nepal 4 Royal Belgian Institute of Natural Sciences, Vautierstraat 29, B-1000, Brussels, Belgium Corresponding author: Prem B. Budha ([email protected]) Academic editor: F. Köhler | Received 17 April 2017 | Accepted 2 May 2017 | Published 23 May 2017 http://zoobank.org/E5C8F163-D615-47B9-8418-CEE8D71A7DAB Citation: Budha PB, Naggs F, Backeljau T (2017) Conchological differentiation and genital anatomy of Nepalese Glessulinae (Gastropoda, Stylommatophora, Subulinidae), with descriptions of six new species. ZooKeys 675: 129– 156. https://doi.org/10.3897/zookeys.675.13252 Abstract Eleven species of Glessulinae belonging to the genera Glessula Martens, 1860 (three species) and Rishetia Godwin-Austen, 1920 (eight species) are reported from Nepal, six of which are new to science and are described here, viz., G. tamakoshi Budha & Backeljau, sp. n., R. kathmandica Budha & Backeljau, sp. n., R. nagarjunensis Budha & Naggs, sp. n., R. rishikeshi Budha & Naggs, sp. n., R. subulata Budha & Naggs and R. tribhuvana Budha, sp. n. and two are new records for Nepal viz. G. cf. hebetata and R. -

Invasive Alien Species in Southern Africa
GISP Global Invasive Species Programme Ministry of Tourism, Environment United States Government and Natural Resources Republic of Zambia Invasive Alien Species in Southern Africa National Reports & Directory of Resources Edited by Ian A.W. Macdonald, Jamie K. Reaser, Chris Bright, Laurie E. Neville, Geoffrey W. Howard, Sean J. Murphy, and Guy Preston 1 This report is a product of a workshop entitled Prevention and Management of Invasive Alien Species: Forging Cooperation throughout Southern Africa, held by the Global Invasive Species Programme (GISP) in Lusaka, Zambia on 10-12 June 2002. It was sponsored by the U.S. Department of State, Bureau of Oceans and International Environmental Affairs (OESI) grant S-LMAQM-00-H-0167. In-kind assistance was provided by the U.S. Environmental Protection Agency. Administrative and logistical assistance was provided by the Zambian Ministry of Tourism, Environment and Natural Resources, the U.S. Embassy in Lusaka, Zambia, the Scientific Committee on Problems of the Environment (SCOPE), and the National Fish and Wildlife Foundation (NFWF), as well as all Steering Committee members. The Smithsonian Institution National Museum of Natural History and National Botanical Institute, South Africa kindly provided support during report production. The editors thank Dr Phoebe Barnard of the GISP Secretariat for her very extensive work to finalize the report. The workshop was co-chaired by the Governments of the Republic of Zambia and the United States of America, and by the Global Invasive Species Programme. Members of the Steering Committee included: Mr Lubinda Aongola (Ministry of Tourism, Environment and Natural Resources, Zambia), Mr Troy Fitrell (U.S. -

Gastropoda: Stylommatophora)1 John L
EENY-494 Terrestrial Slugs of Florida (Gastropoda: Stylommatophora)1 John L. Capinera2 Introduction Florida has only a few terrestrial slug species that are native (indigenous), but some non-native (nonindigenous) species have successfully established here. Many interceptions of slugs are made by quarantine inspectors (Robinson 1999), including species not yet found in the United States or restricted to areas of North America other than Florida. In addition to the many potential invasive slugs originating in temperate climates such as Europe, the traditional source of invasive molluscs for the US, Florida is also quite susceptible to invasion by slugs from warmer climates. Indeed, most of the invaders that have established here are warm-weather or tropical species. Following is a discus- sion of the situation in Florida, including problems with Figure 1. Lateral view of slug showing the breathing pore (pneumostome) open. When closed, the pore can be difficult to locate. slug identification and taxonomy, as well as the behavior, Note that there are two pairs of tentacles, with the larger, upper pair ecology, and management of slugs. bearing visual organs. Credits: Lyle J. Buss, UF/IFAS Biology as nocturnal activity and dwelling mostly in sheltered Slugs are snails without a visible shell (some have an environments. Slugs also reduce water loss by opening their internal shell and a few have a greatly reduced external breathing pore (pneumostome) only periodically instead of shell). The slug life-form (with a reduced or invisible shell) having it open continuously. Slugs produce mucus (slime), has evolved a number of times in different snail families, which allows them to adhere to the substrate and provides but this shell-free body form has imparted similar behavior some protection against abrasion, but some mucus also and physiology in all species of slugs. -

Status of Tree Snails (Gastropoda: Partulidae) on Guam, with a Resurvey of Sites Studied by H
Pacific Science (1992), vol. 46, no. 1: 77-85 © 1992 by University of Hawaii Press. All rights reserved Status of Tree Snails (Gastropoda: Partulidae) on Guam, with a Resurvey of Sites Studied by H. E. Crampton in 19201 DAVID R. HOPPER 2 AND BARRY D. SMITH 2 ABSTRACT: Tree snails of the family Partulidae have declined on Guam since World War II. One species, indigenous to the western Pacific, Partu/a radio/ata, is still locally common along stream courses in southern areas of the island. The Mariana Island endemic Samoanajragilis is present but not found in abundance anywhere on Guam. Partu/a gibba, another Mariana endemic, is currently known only from one isolated coastal valley along the northwestern coast, and appears to be in a state ofdecline. The Guam endemic Partu/a sa/ifana was not found in areas where it had been previously collected by earlier researchers, and is thus believed to be extinct. The decline and extinction ofthese snails are related to human activities. The single most important factor is likely predation by snails that were introduced as biological control agents for the giant African snail, Achatina ju/ica. The current, most serious threat is probably the introduced flatworm P/atydemus manokwari. This flatworm is also the likely cause of extinctions ofother native and introduced gastropods on Guam and may be the most important threat to the Mariana Partulidae. TREE SNAILS OF TROPICAL PACIFIC islands have 1970). With the exception of the partulids of been of interest since early exploration of the Society Islands, all are lacking study. -

Achatina Fulica Background
Giant African Land Snail, Achatina fulica Background • Originally from coastal East Africa and its islands • Has spread to other parts of Africa, Asia, some Pacific islands, Australia, New Zealand, South America, the Caribbean, and the United States • Can be found in agricultural areas, natural forests, planted forests, riparian zones, wetlands, disturbed areas, and even urban areas in warm tropical climates with high humidity • Also known scientifically as Lissachatina fulica • Common names include giant African land snail and giant African snail Hosts Image citation: Cotton - Charles T. Bryson, USDA Agricultural Research Service, www.bugwood.org, #1116132 Banana - Charles T. Bryson, USDA Agricultural Research Service, www.bugwood.org, #1197011 Papaya - Forest & Kim Starr, Starr Environmental, www.bugwood.org, #5420178 Pumpkin - Howard F. Schwartz, Colorado State University, www.bugwood.org, #5365883 Cucumber - Howard F. Schwartz, Colorado State University, www.bugwood.org., #5363704 Carrots - M.E. Bartolo, www.bugwood.org, #5359190 Environmental Impacts • Consumes large quantities and numbers of species of native plants – May cause indirect damage to plants due to the sheer numbers of snails being so heavy that the plants beak under their weight – May also be a vector of several plant pathogens • Outcompetes and may even eat native snails • It eats so much it can alter the nutrient cycling • Their shells can neutralize acid soils and therefore damage plants that prefer acidic soils • Indirectly, the biocontrol and chemical control that is used on this species can affect native snail species as well. Structural Concerns and Nuisance Issues Image citation: Florida Department of Agriculture and Consumer Services, Division of Plant Industry Public Health Concerns • Intermediate host that vectors: – rat lungworm, Angiostrongylus cantonensis (roundworm) – A. -

Abstract Volume
ABSTRACT VOLUME August 11-16, 2019 1 2 Table of Contents Pages Acknowledgements……………………………………………………………………………………………...1 Abstracts Symposia and Contributed talks……………………….……………………………………………3-225 Poster Presentations…………………………………………………………………………………226-291 3 Venom Evolution of West African Cone Snails (Gastropoda: Conidae) Samuel Abalde*1, Manuel J. Tenorio2, Carlos M. L. Afonso3, and Rafael Zardoya1 1Museo Nacional de Ciencias Naturales (MNCN-CSIC), Departamento de Biodiversidad y Biologia Evolutiva 2Universidad de Cadiz, Departamento CMIM y Química Inorgánica – Instituto de Biomoléculas (INBIO) 3Universidade do Algarve, Centre of Marine Sciences (CCMAR) Cone snails form one of the most diverse families of marine animals, including more than 900 species classified into almost ninety different (sub)genera. Conids are well known for being active predators on worms, fishes, and even other snails. Cones are venomous gastropods, meaning that they use a sophisticated cocktail of hundreds of toxins, named conotoxins, to subdue their prey. Although this venom has been studied for decades, most of the effort has been focused on Indo-Pacific species. Thus far, Atlantic species have received little attention despite recent radiations have led to a hotspot of diversity in West Africa, with high levels of endemic species. In fact, the Atlantic Chelyconus ermineus is thought to represent an adaptation to piscivory independent from the Indo-Pacific species and is, therefore, key to understanding the basis of this diet specialization. We studied the transcriptomes of the venom gland of three individuals of C. ermineus. The venom repertoire of this species included more than 300 conotoxin precursors, which could be ascribed to 33 known and 22 new (unassigned) protein superfamilies, respectively. Most abundant superfamilies were T, W, O1, M, O2, and Z, accounting for 57% of all detected diversity. -

Biodiversity in Sub-Saharan Africa and Its Islands Conservation, Management and Sustainable Use
Biodiversity in Sub-Saharan Africa and its Islands Conservation, Management and Sustainable Use Occasional Papers of the IUCN Species Survival Commission No. 6 IUCN - The World Conservation Union IUCN Species Survival Commission Role of the SSC The Species Survival Commission (SSC) is IUCN's primary source of the 4. To provide advice, information, and expertise to the Secretariat of the scientific and technical information required for the maintenance of biologi- Convention on International Trade in Endangered Species of Wild Fauna cal diversity through the conservation of endangered and vulnerable species and Flora (CITES) and other international agreements affecting conser- of fauna and flora, whilst recommending and promoting measures for their vation of species or biological diversity. conservation, and for the management of other species of conservation con- cern. Its objective is to mobilize action to prevent the extinction of species, 5. To carry out specific tasks on behalf of the Union, including: sub-species and discrete populations of fauna and flora, thereby not only maintaining biological diversity but improving the status of endangered and • coordination of a programme of activities for the conservation of bio- vulnerable species. logical diversity within the framework of the IUCN Conservation Programme. Objectives of the SSC • promotion of the maintenance of biological diversity by monitoring 1. To participate in the further development, promotion and implementation the status of species and populations of conservation concern. of the World Conservation Strategy; to advise on the development of IUCN's Conservation Programme; to support the implementation of the • development and review of conservation action plans and priorities Programme' and to assist in the development, screening, and monitoring for species and their populations. -

Gastropoda Pulmonata: Streptaxidae) from the Aldabra Indian Ocean Group, Western
BASTERIA, 50: 71-77, 1986 Additional notes on Gulella (Gastropoda Pulmonata: Streptaxidae) from the Aldabra Indian Ocean group, western A.C. van Bruggen Systematic Zoology Section, Leiden university, c/o Rijksmuseum van Natuurlijke Historie, P.O. Box 9517, 2300 RA Leiden, The Netherlands The availability of new material and reconsideration of types and other specimens has necessitated the publication of the following notes, Acknowledgements are due to Drs. P.B. Mordan of the Mollusca Section, British Museum (Natural History), London (abbreviated BM), and S. Tillier, Laboratoire de Biologie des Invertébrés Marins et de Malacologie, Museum National d’Histoire Naturelle, Paris, for access to material, and to Dr. A.A. Shileyko, Zoological Museum of Moscow State University, Moscow, for depositing some valuable specimens in the Leiden museum. The abbreviation l/d stands for the ratio length/major diameter of shells; this has been calculated from micrometer readings, so that the figures do not with those calculated from the in always agree measurements mm. GULELLA GWENDOLINAE ON COSMOLEDO ISLAND At the Eighth International Malacological Congress in Budapest (1983), Dr. the kind interventionof Dr. I.M. confided the Shileyko, through Likharev, to present author some interesting streptaxid material. These specimens were obtained by Dr. Shileyko during his travels calling at various islands in the western Indian ocean (1983). Among these specimens there are two shells of Gulella gwendolinae (Preston, 1910): "Cosmoledo Island, in leaves under dry Tournefortia sp. [Boraginaceae], 1.IV. 1983, Coll. A.A. Schileyko" (Rijksmuseum van Natuurlijke Historie, Leiden). This may be litt. detailed as Wizard Island of the Cosmoledo group (Shileyko, in 12.V. -

Notiziario S.I.M
NOTIZIARIO S.I.M. Supplemento al Bollettino Malacologico Anno 21 N. 1 – 4 (gennaio – aprile 2003) SOMMARIO Editoriale Vita Sociale Verbale della riunione del Consiglio Direttivo (Cesenatico 23/03/2003) Verbale dell’Assemblea ordinaria di Cesenatico (23/03/2003) Verbale della riunione del Consiglio Direttivo (Prato 10/05/2003) Verbale della riunione del Consiglio Direttivo (Genova 05/07/2003) Comunicazioni delle sezioni locali della S.I.M. o Gruppo Malacologico Milanese o Gruppo Malacologico Campano – Pugliese Elenco delle pubblicazioni S.I.M. disponibili Necrologio: Carlo Cavalieri Chiarelli S. & Micali P. - Vela Luka: lo Ionio in Adriatico. Documenti del Gruppo Malacologico Livornese Le piccole Tricolia mediterranee Segnalazioni bibliografiche Pubblicazioni ricevute Mostra Mercato Scambio, Prato 25-26 ottobre 2003 Recensioni Varie 1 EDITORIALE Cari amici, penso che ormai siate tutti a conoscenza di quanto successo nella S.I.M. a seguito delle ultime elezioni, molti interventi sono apparsi sulla B.B.S., e troverete un ampio resoconto nei verbali pubblicati in questo fascicolo. Non voglio perciò dilungarmi sull’argomento, ma darvi qualche indicazione su quanto stiamo facendo. Sono state recepite le principali osservazioni dei Soci sulla gestione del Bollettino Malacologico, rivista che ha mostrato negli anni una costante crescita qualitativa e che rappresenta ormai un punto fermo (di cui dobbiamo essere orgogliosi) negli studi malacologici mediterranei. Un primo risultato, ossia la presenza di un ampio riassunto in italiano se il lavoro e’ scritto in inglese (e viceversa), sarà già visibile a partire dal prossimo fascicolo stampato. La rivista avrà una struttura ben definita per la sua gestione. Una Direzione Scientifica, affidata a Stefano Schiaparelli, che dovrà garantire sia la scientificità della rivista, sia l’assoluta indipendenza della stessa da condizionamenti “politici”. -

International Conference on Invasive Alien Species Management
Proceedings of the International Conference on Invasive Alien Species Management NNationalational TTrustrust fforor NatureNature ConservationConservation BBiodiversityiodiversity CConservationonservation CentreCentre SSauraha,auraha, CChitwan,hitwan, NNepalepal MMarcharch 2525 – 227,7, 22014014 Supported by: Dr. Ganesh Raj Joshi, the Secretary of Ministry of Forests and Soil Conserva on, inaugura ng the conference Dignitaries of the inaugural session on the dais Proceedings of the International Conference on Invasive Alien Species Management National Trust for Nature Conservation Biodiversity Conservation Centre Sauraha, Chitwan, Nepal March 25 – 27, 2014 Supported by: © NTNC 2014 All rights reserved Any reproduc on in full or in part must men on the tle and credit NTNC and the author Published by : NaƟ onal Trust for Nature ConservaƟ on (NTNC) Address : Khumaltar, Lalitpur, Nepal PO Box 3712, Kathmandu, Nepal Tel : +977-1-5526571, 5526573 Fax : +977-1-5526570 E-mail : [email protected] URL : www.ntnc.org.np Edited by: Mr. Ganga Jang Thapa Dr. Naresh Subedi Dr. Manish Raj Pandey Mr. Nawa Raj Chapagain Mr. Shyam Kumar Thapa Mr. Arun Rana PublicaƟ on services: Mr. Numraj Khanal Photo credits: Dr. Naresh Subedi Mr. Shyam Kumar Thapa Mr. Numraj Khanal CitaƟ on: Thapa, G. J., Subedi, N., Pandey, M. R., Thapa, S. K., Chapagain, N. R. and Rana A. (eds.) (2014), Proceedings of the InternaƟ onal Conference on Invasive Alien Species Management. Na onal Trust for Nature Conserva on, Nepal. This publica on is also available at www.ntnc.org.np/iciasm/publica ons ISBN: 978-9937-8522-1-0 Disclaimer: This proceeding is made possible by the generous support of the Asian Development Bank (ADB), the American people through the United States Agency for InternaƟ onal Development (USAID) and the NaƟ onal Trust for Nature ConservaƟ on (NTNC). -
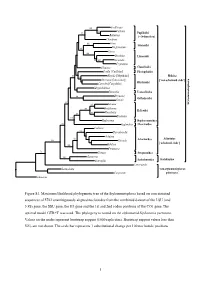
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia