ALTERATION Characferistics OFTHE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Applications for the Post of Night Watchman
List of applications for the post of Night Watchman Receipt Sl. Whether Application is Register Name and address of the applicant No. Accepted (or) Rejected. No. (1) (2) (3) (4) C. Nagarajan, S/o. K.Chellan, 15/15, ElangathuVilai, Palace Road, 1 6373(2) Accepted Thukalay, Kanyakumari District – 629 175. K. Arumugam, S/o. A. Karuppan, 43A/43, Arunthathiyar Street, Age exceeds the maximum 2 6475 Irulappapuram, Kottar Post, Nagercoil. age limit. Hence Rejected. Velayutha Perumal.S., S/o. V. Sudalaiandi, 6C/17, Kulalar Amman Koil Age exceeds the maximum 3 6486 Street, Vagaiadi Street, Kottar, Nagercoil. age limit. Hence Rejected. T. Mahesh, S/o. K. Thalavai, 4/48B, Balavinayagar Koil Street, Thekoor, 4 6497 Accepted Thovalai. D. Jerald, S/o. T. Devaraj, 14-102 L/3, Hookley Garden, Boothapandy Post, 5 6510 Accepted Kanyakumari District-629 852. D.Jebastin, S/o. T.Devaraj, 14-102 L/3, Hookley Garden, Bhoothapandy 6 6518 Accepted Post, Pin 629 852. S. Navaneethan, S/o. S. Saravanan, 3.278C 5/1, Gandhi Colony, 7 6520 Accepted Gurugulam Road, Asaripallam. V. Suresh, S/o. A.Velu, 5/73/4, Ambal Nagar, Kumarapuram, Thoppur, 8 6522 Accepted Kanyakumari District. M. Raju, S/o. S. Murugan, 20A, Manikavasaham Puram, Chettikulam Age exceeds the maximum 9 6532 Junction, Nagercoil. age limit. Hence Rejected A.K. Arul Kumar, 25/1, Pattarkulam Colony, Bhoothapandy Post – 629 852, 10 6589 Accepted Kanyakumari District. S.M. Saravanan, S/o. Sivan Kutty,34/2, 3rd Street, Keezhakulam, 11 6592 Accepted Padmanabhapuram, Thuckalay Post, Kanyakumari District. J. Arun Kumar, S/o. Johnson, Old No.8/41A, New No.8/35, Market Road, 12 6620 Accepted Puliyoorkurichi, Thuckalay Post – 629 175, Kanyakumari District. -

Weathering of Ilmenite from Chavara Deposit and Its Comparison with Manavalakurichi Placer Ilmenite, Southwestern India
Journal of Asian Earth Sciences 34 (2009) 115–122 Contents lists available at ScienceDirect Journal of Asian Earth Sciences journal homepage: www.elsevier.com/locate/jaes Weathering of ilmenite from Chavara deposit and its comparison with Manavalakurichi placer ilmenite, southwestern India Ajith G. Nair a,*, D.S. Suresh Babu a, K.T. Damodaran b, R. Shankar c, C.N. Prabhu d a Centre for Earth Science Studies, PB No. 7250, Akkulam, Thuruvikkal P.O., Thiruvananthapuram 695 031, India b Department of Marine Geology and Geophysics, School of Marine Sciences, Cochin University of Science and Technology, Kochi 682 016, India c Department of Marine Geology, Mangalore University, Mangalagangotri 574 199, India d INETI, Departamento de Geologia Marinha, Estrada da Portela, Zambujal 2720 Alfragide, Portugal article info abstract Article history: The magnetic fractions of ilmenite from the beach placer deposit of Chavara, southwest India have been Received 22 August 2005 studied for mineralogical and chemical composition to assess the range of their physical and chemical Received in revised form 6 February 2006 variations with weathering. Chavara deposit represents a highly weathered and relatively homogenous Accepted 21 March 2008 concentration. Significant variation in composition has been documented with alteration. The most mag- netic of the fractions of ilmenite, separated at 0.15 Å, and with a susceptibility of 3.2 Â 10À6 m3 kgÀ1, indi- cates the presence of haematite–ilmenite intergrowth. An iron-poor, titanium-rich component of the Keywords: ilmenite ore has been identified from among the magnetic fractions of the Chavara ilmenite albeit with Chavara an undesirably high Nb O (0.28%), Cr O (0.23%) and Th (149 ppm) contents. -

Indian Minerals Yearbook 2018 (Part- III : MINERAL REVIEWS)
ZIRCON Indian Minerals Yearbook 2018 (Part- III : MINERAL REVIEWS) 57th Edition ZIRCON (FINAL RELEASE) GOVERNMENT OF INDIA MINISTRY OF MINES INDIAN BUREAU OF MINES Indira Bhavan, Civil Lines, NAGPUR – 440 001 PHONE/FAX NO. (0712) 2565471 PBX: (0712) 2562649, 2560544, 2560648 E-MAIL: [email protected] Website: www.ibm.gov.in November, 2019 29-1 ZIRCON 29 Zircon irconium is the twentieth most abundant RESERVES/RESOURCES Zelement in the Earth's crust and occurs in a Zircon occurs in close association with variety of rock types and geological environment other heavy minerals such as ilmenite, rutile and but most often in the form of zircon (ZrSiO4) monazite in beach sands, along the coastal tracts of found usually as a constituent in heavy mineral the country. Its concentration in the deposits is sand assemblages, which include ilmenite, rutile, about 0.6-18.7% of the total heavy minerals. Indian leucoxene, monazite and garnet in varying zircons analyse 63-66% ZrO2. AMD has carried out proportions. Zircon sand and baddeleyite reconnaissance investigation in parts of Gujarat, Maharashtra, Karnataka, Andhra Pradesh, Tamil (an oxide-ZrO 2) are used via their salts to extract zirconium and hafnium. Normally, all Nadu, Kerala, Odisha and West Bengal during 2008- zirconium compounds contain between 1.4% and 14. The resource estimation in these areas have been carried out up to 2016. The resources of zircon are 3% hafnium. Zircon is very stable at high placed at 33.71 million tonnes as per Department of temperature and has excellent thermal shock Atomic Energy (DAE). The statewise break-up of resistance, low thermal conductivity and the resources is given in Table-1. -

Site-Specific Analysis of Cancer Incidence and Related Sociological Parameters in a ‘Radiation Hot Spot’, Along the West Coast of Tamil Nadu, India
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Site-Specific Analysis of Cancer Incidence and Related Sociological Parameters in a ‘Radiation Hot Spot’, along the West Coast of Tamil Nadu, India Aneesh D1, Kanagappan, M2, Godwin Wesley S3 1Research Scholar, 2Associate Professor, 3Professor Emeritus, Department of Zoology and Research Centre, Scott Christian College (Autonomous), Nagercoil - 629 003, Kanniyakumari District, Tamil Nadu Corresponding Author: Aneesh D ABSTRACT Background: Manavalakurichi, along the west coast of Tamil Nadu, India is well known for its natural radioactivity due to deposition of monazite sand and considered as high background natural radiation area (HBNRA). The incidence of cancer in relation to background radiation and parameters such as occupation, habits (tobacco chewing, smoking, drinking) and gender was preliminarily assessed. Although residing in a HBNRA has been reported to lead to an increase in the frequency of chromosomal aberrations in lymphocytes, the carcinogenic effect has not yet been established. The present study aims to evaluate the impact of background radiation in residents of HBNRA. Materials and Methods: A cross-sectional study was conducted considering 938 and 778 residents from HBNRA and low background natural radiation area (LBNRA), respectively using as health assessment survey questionnaire. Result: The incidence of cancer was 1.49% in HBNRA and 1.43% in LBNRA. Regression analysis of the total population of both areas revealed that radiation dose is not significantly related to incidence of cancer (R2 = 0.010, p=0.411). Pearson chi-square analysis of data showed that a higher proportion (age >18 years) has relationship between cancer and tobacco usage [HBNRA: χ2 (1, N=364) = 7.098, p = 0.009, LBNRA: χ2 (1, N=322) = 5.544, p = 0.024]. -

Kodaiyar River Basin
Kodaiyar River Basin Introduction One of the oldest systems in Tamil Nadu is the “Kodaiyar system” providing irrigation facilities for two paddy crop seasons in Kanyakumari district. The Kodaiyar system comprises the integrated operation of commands of two major rivers namely Pazhayar and Paralayar along with Tambaraparani or Kuzhithuraiyur in which Kodaiyar is a major tributary. The whole system is called as Kodaiyar system. Planning, development and management of natural resources in this basin require time-effective and authentic data.The water demand for domestic, irrigation, industries, livestock, power generation and public purpose is governed by socio – economic and cultural factors such as present and future population size, income level, urbanization, markets, prices, cropping patterns etc. Water Resources Planning is people oriented and resources based. Data relating to geology, geomorphology, hydrogeology, hydrology, climatology, water quality, environment, socio – economic, agricultural, population, livestock, industries, etc. are collected for analysis. For the sake of consistency, other types of data should be treated in the same way. Socio – economic, agricultural and livestock statistics are collected and presented on the basis of administrative units located within this basin area. Location and extent of Kodaiyar Basin The Kodaiyar river basin forms the southernmost end of Indian peninsula. The basin covers an area of 1646.964 sq km. The flanks of the entire basin falls within the TamilnaduState boundary. Tamiraparani basin lies on the north and Kodaiyar basin on the east and Neyyar basin of Kerala State lies on the west. This is the only river basin which has its coastal border adjoining the Arabian sea, the Indian Ocean in the south and the Gulf of Mannar in the east. -

Branch Libraries List
Libraries Address 1 District Central Librarian District Central Library No:61 M S Road, Agasteeswaram Taluk, Nagercoil-629001. Kanyakumari District. Branch Library Address 1 Librarian 8 Librarian 15 Librarian Branch Library Branch Library Branch Library Colachel-629251 Kallukoottam-629808 Kuzhithurai-629163 Market-800, Door No:P.16-71A / 4-120-11 Vilavancode Taluk N.D.N Building, Kalkulam Kalkulam Taluk Taluk Kanyakumari District Kanyakumari District Kanyakumari District 2 Librarian 9 Librarian 16 Librarian Branch Library Branch Library Branch Library Aralvaimozhi-629301 Karankadu-629809 Thirunanthikarai-629161 11-119 Vadakoor Road, Karankadu Post Kulasekaram Post Thovalai Taluk, Kalkulam Taluk, Kanyakumari District Kanyakumari District Kanyakumari District 3 Librarian 10 Libraria n 17 Librarian Branch Library Branch Library Branch Library Manavalakurichi-629252 Monday Market-629802 Sucindram-629704 Door No:12-198, Old No:12-17, Neyyoor & Post Near Panchayat Office, Bech Road Kalkulam Taluk, Marshal Nesamony Park, Kanyakumari District Kanyakumari District Kanyakumari District 4 Librarian 11 Librarian 18 Librarian Branch Library Branch Library Branch Library Agasteeswaram-62701 Painkulam Anjugramam-629401 Door No:14/6, Keel Salai Vilavancode Taluk Police Station Road Post Agasteeswaram Taluk, Kanyakumari District Kanyakumari District Kanyakumari District 5 Librarian 12 Librarian 19 Librarian Branch Library Branch Library Branch Library Azhakappapuram-629401 Arumanai-629151 Kovilanvilai-629252 Door No:12-42 Panchayat Street, Arumanai- Post -

Kanyakumari District, Tamil Nadu
For official use Technical Report Series DISTRICT GROUNDWATER BROCHURE KANYAKUMARI DISTRICT, TAMIL NADU A. Balachandran Scientist - D Government of India Ministry of Water Resources Central Ground Water Board South Eastern Coastal Region Chennai September 2008 0 DISTRICT AT A GLANCE (KANYAKUMARI) S. No. ITEMS STATISTICS 1. GENERAL INFORMATION i. Geographical area (Sq. km) 1671.84 ii. Administrative Divisions (As on 31-3-2007) Number of Taluks 4 Number of Blocks 9 Number of Villages 81 iii. Population (As on 2001 Censes) Total Population 1676034 Male 832269 Female 843765 iv. Average Annual Rainfall (mm) 1448.6 2. GEOMORPHOLOGY i. Major physiographic Units (i). Western Ghats (ii). Coastal Plain. ii. Major Drainages Pazhayar, Valliyar & Tamirabarani. 3. LAND USE (Sq. km) (2005-06) i. Forest area 541.55 ii. Net area sown 793.23 iii. Barren & Uncultivable waste 31.49 4. MAJOR SOIL TYPES 1. Red soil 2. Lateritic soil 3. Clayey soil, 4. River Alluvium & 5. Coastal Alluvium. 5. AREA UNDER PRINCIPAL CROPS (Ha.) 1. Paddy -21709 (56%) (2005-2006) 1. Coconut – 9388 (24%) 2. Banana – 5509 (14.2%) 4. Pulses – 166 (< 1 %) 6. IRRIGATION BY DIFFERENT SOURCES (2005-06) Number Area irrigated (Ha.) i. Dug wells 3349 1535 ii. Tube wells 1303 913 iii. Tanks 2623 13657 iv. Canals 53 22542 vi. Net irrigated area 27694 Ha. vii. Gross irrigated area 38885 Ha. 1 7. NUMBER OF GROUND WATER MONITORING WELLS OF CGWB (As on 31.03.2007) i. Dug wells 14 ii. Piezometers 8 8. PREDOMINANT GEOLOGICAL Recent Alluvium, & Warkalai FORMATIONS Sandstones, Peninsular Gneisses Charnockites, Khondalites, Granites and Pegmatites 9. -
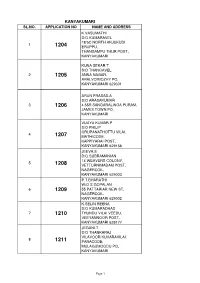
Kanyakumari Sl.No
KANYAKUMARI SL.NO. APPLICATION NO NAME AND ADDRESS K.VASUMATHI D/O KUMARAVEL 18/5C,NORTH ANJUKUDI 1 1204 ERUPPU, THANGAMPU THUR POST, KANYAKUMARI KUNA SEKAR.T S/O THANGAVEL 2 1205 ANNA NAGAR, ARALVOIMOZHY PO, KANYAKUMARI 629301 ARUN PRASAD.A S/O ARASAKUMAR 3 1206 4.55R SANGARALINGA PURAM, JAMES TOWN PO, KANYAKUMARI VIJAYA KUMAR.P S/O PHILIP ORUPANATHOTTU VILAI, 4 1207 MATHICODE, KAPPIYARAI POST, KANYAKUMARI 629156 JEEVA.S D/O SUBRAMANIAN 14 WEAVERS COLONY, 5 1208 VETTURNIMADAM POST, NAGERCOIL, KANYAKUMARI 629003 P.T.BHARATHI W/O S.GOPALAN 6 1209 55 PATTARIAR NEW ST, NAGERCOIL, KANYAKUMARI 629002 K.SELIN REENA D/O KUMARADHAS 7 1210 THUNDU VILAI VEEDU, VEEYANNOOR POST, KANYAKUMARI 629177 JEGANI.T D/O THANKARAJ VILAVOOR KUVARAVILAI, 8 1211 PARACODE, MULAGUMOODU PO, KANYAKUMARI Page 1 FELSY FREEDA.L D/O LAZER 9 1212 MALAANTHATTU VILAI, PALLIYADI POST, KANYAKUMARI 629169 CHRISTAL KAVITHA. R D/O RAJAN 10 1213 VALIYAVILAGAM HOUSE ST, MANKADU POST, KANYAKUMARI 629172 PRABHA.P D/O PADMANABHAN 11 1214 EATHENKADU, FRIDAYMARKET POST, KANYAKUMARI 629802 KALAI SELVI.N D/O NARAYANA PERUMAL 12 1215 THERIVILAI SWAMITHOPPU POST, KANYAKUMARI 629704 NAGALAKSHMI.M D/O MURUGESAN LEKSHMI BHAVAN, 13 1216 CHAKKIYANCODE, NEYYOOR POST, KANYAKUMARI 629802 BEULA.S D/O SATHIADHAS 14 1217 ANAN VILAI, KEEZHKULAM PO, KANYAKUMARI 629193 MAHESWARI.S D/O SIVACHANDRESWARAN 15 1218 1/20BTHEKKURICHI, RAJAKKAMANGALAM POST, KANYAKUMARI 629503 PREMALATHA.S W/O MURALIRAJ V.L, 460F-1, M.S.ROAD, 16 1219 SINGARATHOPPUPAR, VATHIPURAM, NAGERCOIL, KANYAKUMARI 629003 SUBASH.T S/O THANKAPPAN MANALI KATTU VILAI, 17 1220 PUTHEN VEEDU THICKA, NAMCODE PO, KANYAKUMARI 629804 Page 2 J. -

I INDIAN RARE EARTHS LTD Manavalakurichi
I INDIAN RARE EARTHS LTD (A Govt. of India Undertaking) Manavalakurichi - 629 252 Kanyakumari District, Tamil Nadu. Phone (04651) 237255-257 Fax: (04651)-237220 e-mail: [email protected] , website: www.irel.gov.in Invitation to Tender TENDER NO.MK/P.64-5/2009-10/37/ November 20, 2009 Sealed offers are invited by Head, IRE Ltd., Manavalakurichi from experienced, reliable and bonafide Contractors/ Firms/ Companies for the following work with superscription as to Tender No., due date, etc., In the absence of superscription on the envelope with reference to the above, offer is liable to be rejected. 1. Description of work : Annual maintenance contract for air tables 2. Last date and time for : 17.12.2009 2.15 PM. receipt of tender offer(s) 3. Date and time for opening of : 17.12.2009 2.30 PM. tender offers 4. Earnest money Deposit : Nil 5. Cost of tender form : Nil 6. Period of contract : 1 year 7. Payment terms : Payment shall be made once in a month for the completed work. 8. Enclosures to the tender : Annexure I – Scope of work with other conditions and Ethics & Undertaking. Annexure II – General Terms & conditions Annexure III - Price schedule 9. Tenders may be posted by Registered Post with due allowance for postal delay. Late tenders are liable to be rejected. Offers by way of telegrams and fax/e.mail are not acceptable. 10. Tender(s) will be opened on the date and time noted above in the presence of the tenderer(s) who may choose to be present. 11. Tender forms are NOT TRANSFERABLE. -

Kanyakumari District
KANYAKUMARI DISTRICT 1 KANYAKUMARI DISTRICT 1. Introduction i) Geographical location of the district Kanyakumari is the Southern most West it is bound by Kerala. With an area of district of Tamil Nadu. The district lies 1672 sq.km it occupies 1.29% of the total between 77 o 15 ' and 77 o 36 ' of the Eastern area of Tamil Nadu. It ranks first in literacy Longitudes and 8 o 03 ' and 8 o 35 ' of the among the districts in Tamil Nadu. Northern Latitudes. The district is bound by Tirunelveli district on the North and the East. ii) Administrative profile The South eastern boundary is the Gulf of The administrative profile of Mannar. On the South and the South West, Kanyakumari district is given in the table the boundaries are the Indian Ocean and the below Arabian sea. On the west and North Name of the No. of revenue Sl. No. Name of taluk No. of firka division villages 1 Agastheeswaram 4 43 1 Nagercoil 2 Thovalai 3 24 3 Kalkulam 6 66 2 Padmanabhapuram 4 Vilavancode 5 55 Total 18 188 ii) 2 Meteorological information and alluvial soils are found at Based on the agro-climatic and Agastheeswaram and Thovalai blocks. topographic conditions, the district can be divided into three regions, namely: the ii) Agriculture and horticulture uplands, the middle and the low lands, which are suitable for growing a number of crops. Based on the agro-climatic and The proximity of equator, its topography and topographic conditions, the district can be other climate factors favour the growth of divided into three regions, namely:- various crops. -

Kanyakumari District Statistical Handbook – 2016
Kanyakumari District Statistical Handbook – 2016 Preface Salient Features District Profile 1. Area &Population 2. Climate & Rainfall 3. Agriculture 4. Irrigation 5. Animal Husbandary 6. Banking & Insurance 7. Co-Operative Societies 8. Civil Supplies 9. Communications 10. Electricity 11. Education 12. Fisheries 13. Handloom 14. Handicrafts 15. Health & Family Welfare 16. Housing 17. Industries 18. Factories 19. Local Bodies 20. Labour & Employment 21. Legal services 22. Libraries 23. Mining & Quarrying 24. Manufacturing 27. Non-Conventional 25. Medical Services 26. Motor Vehicles Energy 28. Police & Prison 29. Public Health 30. Printing & Publications 31. Prices Indices 32. Quality Control 33. Registration 36. Recreation & Cultural 34. Repair & Services 35. Restaurants & Hotels Services 39. Scientific Research 37. Social Welfare 38. Sanitary Services Services 40. Storage Facilities 41. Textiles 42. Trade & Commerce 43. Transport 44. Tourism 45. Vital Statistics 46. Voluntary Services 47. Waterworks & Supply 48. Rubber Study DEPUTY DIRECTOR OF STATISTICS KANNIYAKUMARI DISTRICT PREFACE The District Statistical Hand Book is prepared and published by our Department every year. This book provides useful data across various departments in Kanniyakumari District. It contains imperative and essential statistical data on different Socio-Economic aspects of the District in terms of statistical tables and graphical representations. This will be useful in getting a picture of Kanniyakumari’s current state and analyzing what improvements can be brought further. I would liketo thank the respectable District Collector Sh. SAJJANSINGH R CHAVAN, IAS for his cooperation in achieving the task of preparing the District Hand Book for the year 2015-16 and I humbly acknowledge his support with profound gratitude. The co-operation extended by the officers of this district, by supplying the information presented in this book is gratefully acknowledged. -

Kanyakumari District
Kanyakumari District Statistical Handbook 2010-11 1. Area & Population 2. Climate & Rainfall 3. Agriculture 4. Irrigation 5. Animal Husbandary 6. Banking & Insurance 7. Co-operation 8. Civil Supplies 9. Communications 10. Electricity 11. Education 12. Fisheries 13 Handloom 14. handicrafts 15. Health & Family Welfare 16. Housing 17. Industries 18. Factories 19. Legal Bodies 20. Labour&Employment 21. Legal Services 22. Libraries 23. Mining & Quarrying 24. Manufacturing 25. Medical services 26 Motor Vehicles 27. NonConventional Energy 28. Police & Prison 29. Public Health 30. Printing & publication 31. Price Indices. 32. Quality Control 33. Registration 34. Repair & Services 35. Restaurents & Hotels 36. Recreation 37. Social Welfare 38. Sanitary services 39. Scientific Research 40. Storage Facilities 41 Textiles 42. Trade & Commerce 43. Transport 44. Tourism 45. Birth & Death 46.Voluntary Services 47. Waterworks & Supply 1 1.AREA AND POPULATION 1.1 AREA, POPULATION, LITERATES, SC, ST – SEXWISE BY BLOCKS YEAR: 2010-2011 Population Literate Name of the Blocks/ Sl.No. Municipalities Male Male Female Female Persons Persons Area (sq.km) 1 2 3 4 5 6 7 8 9 1 Agastheswaram 133.12 148419 73260 75159 118778 60120 58658 2 Rajakkamangalam 120.16 137254 68119 69135 108539 55337 53202 3 Thovalai 369.07 110719 55057 55662 85132 44101 41031 4 Kurunthancode 106.85 165070 81823 83247 126882 64369 62513 5 Thuckalay 130.33 167262 82488 84774 131428 66461 64967 6 Thiruvattar 344.8 161619 80220 81399 122710 62524 60186 7 Killiyoor 82.7 156387 78663 77724 119931