Comparative Performance Evaluation of Horro and Menz Sheep of Ethiopia Under Grazing and Intensive Feeding Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Local History of Ethiopia an - Arfits © Bernhard Lindahl (2005)
Local History of Ethiopia An - Arfits © Bernhard Lindahl (2005) an (Som) I, me; aan (Som) milk; damer, dameer (Som) donkey JDD19 An Damer (area) 08/43 [WO] Ana, name of a group of Oromo known in the 17th century; ana (O) patrikin, relatives on father's side; dadi (O) 1. patience; 2. chances for success; daddi (western O) porcupine, Hystrix cristata JBS56 Ana Dadis (area) 04/43 [WO] anaale: aana eela (O) overseer of a well JEP98 Anaale (waterhole) 13/41 [MS WO] anab (Arabic) grape HEM71 Anaba Behistan 12°28'/39°26' 2700 m 12/39 [Gz] ?? Anabe (Zigba forest in southern Wello) ../.. [20] "In southern Wello, there are still a few areas where indigenous trees survive in pockets of remaining forests. -- A highlight of our trip was a visit to Anabe, one of the few forests of Podocarpus, locally known as Zegba, remaining in southern Wello. -- Professor Bahru notes that Anabe was 'discovered' relatively recently, in 1978, when a forester was looking for a nursery site. In imperial days the area fell under the category of balabbat land before it was converted into a madbet of the Crown Prince. After its 'discovery' it was declared a protected forest. Anabe is some 30 kms to the west of the town of Gerba, which is on the Kombolcha-Bati road. Until recently the rough road from Gerba was completed only up to the market town of Adame, from which it took three hours' walk to the forest. A road built by local people -- with European Union funding now makes the forest accessible in a four-wheel drive vehicle. -
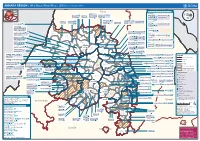
AMHARA REGION : Who Does What Where (3W) (As of 13 February 2013)
AMHARA REGION : Who Does What Where (3W) (as of 13 February 2013) Tigray Tigray Interventions/Projects at Woreda Level Afar Amhara ERCS: Lay Gayint: Beneshangul Gumu / Dire Dawa Plan Int.: Addis Ababa Hareri Save the fk Save the Save the df d/k/ CARE:f k Save the Children:f Gambela Save the Oromia Children: Children:f Children: Somali FHI: Welthungerhilfe: SNNPR j j Children:l lf/k / Oxfam GB:af ACF: ACF: Save the Save the af/k af/k Save the df Save the Save the Tach Gayint: Children:f Children: Children:fj Children:l Children: l FHI:l/k MSF Holand:f/ ! kj CARE: k Save the Children:f ! FHI:lf/k Oxfam GB: a Tselemt Save the Childrenf: j Addi Dessie Zuria: WVE: Arekay dlfk Tsegede ! Beyeda Concern:î l/ Mirab ! Concern:/ Welthungerhilfe:k Save the Children: Armacho f/k Debark Save the Children:fj Kelela: Welthungerhilfe: ! / Tach Abergele CRS: ak Save the Children:fj ! Armacho ! FHI: Save the l/k Save thef Dabat Janamora Legambo: Children:dfkj Children: ! Plan Int.:d/ j WVE: Concern: GOAL: Save the Children: dlfk Sahla k/ a / f ! ! Save the ! Lay Metema North Ziquala Children:fkj Armacho Wegera ACF: Save the Children: Tenta: ! k f Gonder ! Wag WVE: Plan Int.: / Concern: Save the dlfk Himra d k/ a WVE: ! Children: f Sekota GOAL: dlf Save the Children: Concern: Save the / ! Save: f/k Chilga ! a/ j East Children:f West ! Belesa FHI:l Save the Children:/ /k ! Gonder Belesa Dehana ! CRS: Welthungerhilfe:/ Dembia Zuria ! î Save thedf Gaz GOAL: Children: Quara ! / j CARE: WVE: Gibla ! l ! Save the Children: Welthungerhilfe: k d k/ Takusa dlfj k -

Abbysinia/Ethiopia: State Formation and National State-Building Project
Abbysinia/Ethiopia: State Formation and National State-Building Project Comparative Approach Daniel Gemtessa Oct, 2014 Department of Political Sience University of Oslo TABLE OF CONTENTS No.s Pages Part I 1 1 Chapter I Introduction 1 1.1 Problem Presentation – Ethiopia 1 1.2 Concept Clarification 3 1.2.1 Ethiopia 3 1.2.2 Abyssinia Functional Differentiation 4 1.2.3 Religion 6 1.2.4 Language 6 1.2.5 Economic Foundation 6 1.2.6 Law and Culture 7 1.2.7 End of Zemanamesafint (Era of the Princes) 8 1.2.8 Oromos, Functional Differentiation 9 1.2.9 Religion and Culture 10 1.2.10 Law 10 1.2.11 Economy 10 1.3 Method and Evaluation of Data Materials 11 1.4 Evaluation of Data Materials 13 1.4.1 Observation 13 1.4.2 Copyright Provision 13 1.4.3 Interpretation 14 1.4.4 Usability, Usefulness, Fitness 14 1.4.5 The Layout of This Work 14 Chapter II Theoretical Background 15 2.1 Introduction 15 2.2 A Short Presentation of Rokkan’s Model as a Point of Departure for 17 the Overall Problem Presentation 2.3 Theoretical Analysis in Four Chapters 18 2.3.1 Territorial Control 18 2.3.2 Cultural Standardization 18 2.3.3 Political Participation 19 2.3.4 Redistribution 19 2.3.5 Summary of the Theory 19 Part II State Formation 20 Chapter III 3 Phase I: Penetration or State Formation Process 20 3.0.1 First: A Short Definition of Nation 20 3.0.2 Abyssinian/Ethiopian State Formation Process/Territorial Control? 21 3.1 Menelik (1889 – 1913) Emperor 21 3.1.1 Introduction 21 3.1.2 The Colonization of Oromo People 21 3.2 Empire State Under Haile Selassie, 1916 – 1974 37 -

Behavior of Geladas and Other Endemic Wildlife During a Desert Locust Outbreak at Guassa, Ethiopia: Ecological and Conservation Implications
Primates (2010) 51:193–197 DOI 10.1007/s10329-010-0194-6 NEWS AND PERSPECTIVES Behavior of geladas and other endemic wildlife during a desert locust outbreak at Guassa, Ethiopia: ecological and conservation implications Peter J. Fashing • Nga Nguyen • Norman J. Fashing Received: 27 January 2010 / Accepted: 16 February 2010 / Published online: 24 March 2010 Ó Japan Monkey Centre and Springer 2010 Abstract Desert locust (Schistocerca gregaria) outbreaks locust control strategies and potential unintended adverse have occurred repeatedly throughout recorded history in effects on endemic and endangered wildlife. the Horn of Africa region, devastating crops and contrib- uting to famines. In June 2009, a desert locust swarm Keywords Desert locust Á Diet Á Ethiopian wolf Á invaded the Guassa Plateau, Ethiopia, a large and unusually Gelada Á Global warming Á Guassa Á Locust control Á intact Afroalpine tall-grass ecosystem, home to important Pesticide Á Thick-billed raven populations of geladas (Theropithecus gelada), Ethiopian wolves (Canis simensis), thick-billed ravens (Corvus crassirostris), and other Ethiopian or Horn of Africa en- Introduction demics. During the outbreak and its aftermath, we observed many animals, including geladas, ravens, and a wolf, Desert locusts (Schistocerca gregaria) have a long history feeding on locusts in large quantities. These observations of devastating crops and contributing to famines across suggest surprising flexibility in the normally highly spe- southwest Asia, the Middle East, and northern Africa (van cialized diets of geladas and wolves, including the potential Huis 1995). Generally solitary and occurring at low den- for temporary but intensive insectivory during locust out- sities, desert locusts occasionally become gregarious fol- breaks. -

Women and Warfare in Ethiopia
ISSN 1908-6295 Women and Warfare in Ethiopia Minale Adugna Gender Issues Research Report Series - no. 13 Organization for Social Science Research in Eastern and Southern Africa Women and Warfare in Ethiopia A Case Study of Their Role During the Campaign of Adwa, 1895/96, and the Italo-Ethiopian War, 1935-41 Minale Adugna Organization for Social Science Research in Eastern and Southern Africa Gender Issues Research report Series - no. 13 CONTENTS Preface ............................................................................................................... v Acknowledgements............................................................................................ vi Abstract ............................................................................................................. 1 1. Women and War in Ethiopia: From Early Times to the Late 19th Century 1 1.1 The Role of Women in Mobilization ...................................................... 2 1.2 The Role of Women at Battlefields ........................................................ 7 2. The Role of Women during the Campaign of Adwa, 1895/96 ......................... 13 2.1 Empress Taitu and the Road to Adwa .................................................... 13 2.2 The Role of Women at the Battle of Adwa ............................................ 19 3. The Ethiopian Women and the Italio-Ethiopian War, 1935-41 ........................ 21 4. The Impact of War on the Life of Ethiopian Women ....................................... 33 References ........................................................................................................ -

AFTER Rhile DERG: an ASSESSMENT of RURAL LAND
AFTERrHilE DERG: AN ASSESSMENT OF RURAL LAND TENURE ISSUES IN ETHIOPIA by John W. Bruce Land Tenure Center, University of Wisconsin-Madison Allan Hoben African Studies Center, Boston University Dessalegn Rahmato Institute of Development Research, Addis Ababa University A collaborative project of the Land Tenure Center, University of Wisconsin- Madison, and the Institute of Development Research, Addis Ababa University, funded by the Ford Foundation. March 1994 AN ARSSI PEASANT'S REFLECTIONS Before the Derg we were servants. The Derg gave us land, but took away our children. When the cooperative came we did not have rest or the right to market our crops. Today is good. Everyone has his own land and can market his own crops. Our only problem now is that there is not enough land. iii TABLE OF CONTENTS List of boxes Vii List of acronyms ix Preface xi Executive summary xiii 1. Background and context 1 1.1 Prerevolutionary land tenure 1 1.2 Agrarian reform 2 1.3 Rural institutions 6 1.3.1 Peasant associations 6 1.3.2 Producers cooperatives 7 1.3.3 Service cooperatives 7 1.3.4 Current situation of rural institutions 8 1.4 Postrevolutionary land tenure policy 9 2. Current situation 11 2.1 Smallholder agriculture 11 2.1.1 Role of smallholder agriculture 11 2.1.2 Access to agricultural land 12 2.1.3 Transactions and strategies 19 2.1.4 Redistribution by the TPLF and EPRDF: pressures and pros- pects 22 2.1.5 Fragmentation and subdivision 28 2.1.6 Security of tenure and investment 30 2.2 Other production contexts 32 2.2.1 Land endowments 32 2.2.2 Large-scale commercial agriculture and access to land 32 2.2.3 State farms 36 2.2.4 Community forestry 37 2.2.5 Community pasture 38 2.2.6 Pastoralist land tenure 39 2.3 Making land policy and administering land 42 2.3.1 Current situation 42 2.3.2 Leadership and decentralization 44 2.3.3 Land registration and taxation 46 3. -

The Role of Rural Land Registration and Certification Program in Ensuring
International Journal of Agricultural Extension and Rural Development Studies Vol.2, No.2, pp.44-52, October 2015 ___Published by European Centre for Research Training and Development UK (www.eajournals.org) THE ROLE OF RURAL LAND REGISTRATION AND CERTIFICATION PROGRAM IN ENSURING TENURE SECURITY IN MENZ GERA MIDIR DISTRICT, AMHARA STATE, ETHIOPIA Ayele Behaylu1*, Amare Bantider2, Abineh Tilahun1 and Temesgen Gashaw3 1Department of Geography and Environmental Studies, Adigrat University, Ethiopia 2Center for Food Security Studies, College of Development Studies, Addis Abeba University, Ethiopia 3Center for Environmental Science, College of Natural Sciences, Addis Abeba University, Ethiopia ABSTRACT: Land registration and certification has been perceived as a precondition for secure property rights and agricultural development. The objective of this study was to examine the role of rural land registration and certification program in ensuring tenure security in Menz Gera Midir District, in Amhara State. Data for this study were collected through questionnaire, interview of farmers and experts of the field and focus group discussions. About four hundred households were taken as sample population for the questionnaire. A total of one hundred thirty two households were participated in the focus group discussions. The data collected through questionnaire were analyzed quantitatively; whereas data collected through focus group discussions and interviews were compiled, summarized and interpreted qualitatively by cross checking with responses of questionnaires. The findings of this study show that in Menz Gera Midir District rural land registration and certification program ensured tenure security. KEYWORDS: Rural Land Registration and Certification, Land Investments, Tenure Security INTRODUCTION Billions of people especially rural dwellers in developing countries of the world based their lives on land. -

Contribution of the Guassa Community Eco-Lodge to the Conservation of Wild Mammals, Birds and Woody Plants in Menz-Gera Midir Di
International Journal of Avian & Wildlife Biology Research Article Open Access Contribution of the guassa community eco-lodge to the conservation of wild mammals, birds and woody plants in menz-gera midir district, north shewa administrative zone, Ethiopia Abstract Volume 2 Issue 4 - 2017 In recent decades, several eco-lodges have been established in biodiversity areas Solomon Ayele Tadesse,1 Demel Teketay2 of Ethiopia, but quantitative scientific information that helps guide the sustainable 1Department of Natural Resources Management, Debre Berhan management of eco-lodges is lacking. This study was the first attempt that examined University, Ethiopia the role of the GCEL for the conservation of wild mammals, birds, and woody plants. 2Department of Crop Science and Production, Botswana The objective of the study was to characterize and quantify species diversities and University of Agriculture and Natural Resources, Botswana densities of wild mammals, birds, and woody plants in the compound of the eco-lodge and in the adjacent field. Following a total of six randomly aligned transects, field Correspondence: Solomon Ayele Tadesse, Department of data on the species richness and abundance of wild mammals and birds across the Natural Resources Management, College of Agriculture and landscape were collected in the compound of the GCEL (here after the EHH) versus in Natural Resource Sciences, Debre Berhan University, PO Box the adjacent field (here after the FGH). 445, Debre Berhan, Ethiopia, Tel +251-111-6815440, +251-946- 703660, Fax +251-111-6812065, Moreover, a systematic sampling design was employed to lay a total of 39 circular Email [email protected] plots (i.e, each with a radius of 10m) and collect data on the species richness and abundance of woody plants. -

A Case Study from the Guassa Area of Menz, Ethiopia
The Resilient Nature of Common Property Resource Management Systems: a case study from the Guassa area of Menz, Ethiopia Zelealem Tefera Ashenafi1,2 and N. Leader-Williams2 Abstract Many communities world-wide face serious environmental degradation, including deforestation, overgrazing, soil erosion, overexploitation of biodiversity and serious air and water pollution problems, all associated with mismanagement of natural resources. However, natural resource management institutions that are based on systems of common property can often prevent many instances of mismanagement of natural resources. To this end this paper examines how a common property resource management system in the Central Highlands of Ethiopia survived various government sponsored development packages and social changes. In the district of Menz, the Guassa area, common property resource management worked under an indigenous resource management institution known us the Qero system, based on the existing Atsme Irist indigenous land tenure system. The rules of exclusion governing access to the use of the Guassa area resource were aspects of the Atsme Irist land tenure system that conferred usufruct right on the members of a group tracing their lineage back to their pioneer fathers. Furthermore, the user community was organised at parish level, an arrangement that gave the Guassa area the status of consecrated land, under protective patronage of the Christian church in Ethiopia. Following the 1974 Socialist Revolution in Ethiopia, the then governing regime proclaimed the Agrarian Reform in 1975. All land that was in private ownership or communal tenure was transformed into the state or public land tenure system. In turn, this result in the formal ending of the Qero system in Menz. -

Draft Outline
Livelihoods for Resilience Learning Activity Quarterly Report July 1, 2018 to September 30, 2018 Submission Date: October 30, 2018 Leader with Associates Cooperative Agreement Number: AID-OAA-A-10-00006 Associate Cooperative Agreement Number 72066318LA00001 Activity Start and End Dates: September 2, 2016 to September 1, 2021 Submitted To: Endale Lemma Submitted by: Tom Spangler Save the Children Federation, Inc. 899 North Capitol Street, NE Washington, DC 20002 Tel: 202-640-6600 Email: [email protected] 1 This document was produced for review by the United States Agency for International Development. TABLE OF CONTENTS Table of Contents ..................................................................................................................................... i List of Abbreviations and Acronyms .............................................................................................. ii 1. PROGRAM OVERVIEW/SUMMARY ............................................................................................... 1 1.1 Program Description .................................................................................................................... 1 Background ...................................................................................................................................... 1 Objective of the Livelihoods for Resilience Learning Activity ........................................................... 2 2. ACTIVITY IMPLEMENTATION PROGRESS ............................................................................... -

On-Farm Pre-Weaning Growth Performance of Washera, Farta and Their Crossbred Lambs in Selected Districts of Western Amhara Region, Ethiopia
Ethiop. J. Sci. & Technol. 11(3): 253-270, 2018 253 On-farm pre-weaning growth performance of Washera, Farta and their crossbred lambs in selected districts of western Amhara Region, Ethiopia Esubalew Adimasu1,*, Kefyalew Alemayehu1 , and Tesfaye Getachew2 1Bahir Dar University, Department of Animal Production and Technology, College of Agriculture and Environmental Science, P.O. Box 5501, Bahir Dar, Ethiopia 2International Center for Agricultural Research in the Dry Areas (ICARDA), Addis Ababa, Ethiopia ABSTRACT The objective of the study was to evaluate the pre-weaning growth performance of Farta, Washera, and their cross-sheep lambs. The study was conducted in Farta and Lay Gayint districts of the Amhara National Regional State. The data were collected from November 2016 to May 2017. A total of 132 sheep, i.e., 82 Farta, 20 Washera, and 30 Washera-Farta crossbred, were selected using purposive sampling technique to assess their pre-weaning growth performance. Growth data was analyzed using SAS Version 9.1.3. (SAS, 2008). Mean birth weight (± SE in kg) was 2.7±0.30 for Farta, 3.1±0.14 for Washera, and 2.9±0.08 for Washera-Farta crossbred lambs. Weaning weights were 10.9±0.30 kg for Farta, 13.1±0.50 kg for Washera, and 12.2±1.1 kg for Washera-Farta crossbreds. Daily weight gain calculated from birth to 30 days was 89.4±6.44 g for Farta, 119.4±23.53 g for Washera and 111.4±10.46 g for Washera-Farta crossbreds. Weight gain from birth to 90 days was 88.8±3.21 g, 103.2±11.75 g, and 111.9±5.22 g, respectively. -

Local History of Ethiopia : Facha
Local History of Ethiopia Facha - Fyanja © Bernhard Lindahl (2008) facha, fachcha (O) 1. trophy such as a buffalo tail; 2. gadfly; facha-u (O) be scattered HDD93c Facha, village at the main road near Ambo 08/37? [x] JC... Facha (Faccia, Ciaffe) (small village) c.3000 m 06/40 [+ Gu] fache (O) curved sword HCE96c Fachena (with rock shelter), in Aroresa wereda 06/38 [n] fachi: faki (faqi) (A,O) tanner, dresser of skins; fakki (O) comb; fakke (faqqee) (O) gap-toothed HCG69 Fachi 0654'/3540' 1911 m 06/35 [Gu Gz] facho (A) buffalo's tail as a trophy of hunt HDM60 Facho (Faccio) (area) 09/39 [+ WO] JCA99 Facho (Faccio) 06/40 [+ WO] HES34 Facoc, see Fakoch -- Fadashi, ethnic group (sub-group related to the Jebelawi) GDM74 Fadasi, see Bambesi HD... Fadasi, see Gorgura for more recent time faddi (Som) kind of gift JBU50 Faddi ../.. [..] fade (O) helped, cured HDK97 Fade, see Fale JBP49c Fader Guml 04/41 [Wa] fadi: faddi (Som) kind of gift; fadhi (Som) sit, be seated, reside; meeting HFF90 Fadi (Fekado, Fik'ada Fada) 14/39 [Gz] 1424'/3923' 2519 m -- Fadiro language, see Bambassi -- Fadis, Fidis, name of an Ania tribe of eastern Oromo JDC95 Fadis (Faddis) (wide area), cf Fedis 09/42 [WO Gu] (24 km by road from Harar) fado: faddo (Som) wilderness HDN54 Fado (Fadoc) (area) 1029'/3507 825 m 10/35 [WO Gz] Coordinates would give map code HDN53 GD... Fadogno (in the Asosa region) 10/34? [n] GDU54 Fadong (hill) 10/34 [Mi] in the Bomo area about 50 km NNE of Asosa faf: faaf (Som) being spread about JCF05 Faf (wide depression) 05/44 [WO] JCM10 Faf 0629'/4418' 459 m 06/44 [WO 18 Gz] JDH61 Faf, G.