Low Wnt/B-Catenin Signaling Determines Leaky Vessels in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Near-Infrared Chemical Imaging and the PAT Initiative NIR-CI Adds a Completely New Dimension to Conventional NIR Spectroscopy
Molecular Spectroscopy Workbench Near-infrared Chemical Imaging and the PAT Initiative NIR-CI adds a completely new dimension to conventional NIR spectroscopy. E. Neil Lewis, Joe Schoppelrei, and Eunah Lee Dear and gentle readers, this month I present an important other manufacturing steps have on the final dosage form? To tool in the process analytical technologies (PAT) initiative: encourage the PAT initiative FDA is streamlining the mecha- Near-infrared chemical imaging (NIR-CI). You might wonder nism for adopting new technologies in pharmaceutical manu- why I am seemingly concentrating on IR and NIR. The an- facturing. swer is quite simple: I need all the heat I can get, here in New York. Seriously, E. Neil Lewis, Joe Schoppelrei, and The Role of Near-infrared Chemical Imaging (NIR-CI) Eunah Lee of Spectral Dimensions (Olney, MD) have put to- A typical tablet is not just a pressed block of a single material, gether an excellent explanation of what NIR-CI is and what but rather a complex matrix containing one or more active its part in PAT is and will be. For those of you who do not pharmaceutical ingredients (APIs), fillers, binders, disinte- know Neil Lewis, he is one of the pioneers in IR and NIR im- grants, lubricants, and other materials. A basic problem in aging. He has won numerous awards for his research at the pharmaceutical manufacturing is that a relatively simple for- National Institutes of Health (NIH) and subsequent accom- mulation with identical ingredients can produce widely vary- plishments. It is truly exciting to have this paper in my hum- ing therapeutic performance depending upon how the ingre- ble column and I know you will find it informative and enter- dients are distributed in the final matrix. -

Comprehensive Bibliography on Martian Meteorites (Compiled by C
Comprehensive Bibliography on Martian Meteorites (compiled by C. Meyer, March 2008) Abu Aghreb A.E., Ghadi A.M., Schlüter J., Schultz L. and Thiedig F. (2003) Hamadah al Hamra and Dar al Gani: A comparison of two meteorite fields in the Libyan Sahara (abs). Meteoritics & Planet. Sci. 38, A48. Agee Carl B. (2002) Garnet and majorite fractionation in the early Earth and Mars (abs#1862). Lunar Planet. Sci. XXXIII Lunar Planetary Institute, Houston. (CD-ROM). (see address of LPI in Appendix III) Agee C.B., Bogard Don D., Draper D.S., Jones J.H., Meyer Chuck and Mittlefehldt D.W. (2000) Proposed science requirements and acquisition priorities for the first Mars sample return (abs). In Concept and Approaches for Mars Exploration. Part 1 (ed. S. Hubbard) LPI Contribution # 1062. Lunar Planetary Institute, Houston. Agee C.B. and Draper Dave S. (2003) Melting of model Martian mantle at high pressure: Implications for the composition of the Martian basalt source region (abs#1408). Lunar Planet. Sci. Conf. 34th, Lunar Planetary Institute, Houston (CD-ROM). Agerkvist D.P. and Vistisen L. (1993) Mössbauer spectroscopy of the SNC meteorite Zagami (abs). Lunar Planet. Sci. XXIV, 1-2. Lunar Planetary Institute, Houston. Zagami Agerkvist D.P., Vistisen L., Madsen M.B. and Knudsen J.M. (1994) Magnetic properties of Zagami and Nakhla (abs). Lunar Planet. Sci. XXV, 1-2. Lunar Planetary Institute, Houston. Zagami Nakhla Akai J. (1997) Characteristics of iron-oxide and iron-sulfide grains in meteorites and terrestrial sediments, with special references to magnetite grains in Allan Hills 84001 (abs). Meteoritics & Planet. Sci. -

Army Acquisition Workforce Dependency on E-Mail for Formal
ARMY ACQUISITION WORKFORCE DEPENDENCY ON E-MAIL FOR FORMAL WORK COORDINATION: FINDINGS AND OPPORTUNITIES FOR WORKFORCE PERFORMANCE IMPROVEMENT THROUGH E-MAIL-BASED SOCIAL NETWORK ANALYSIS KENNETH A. LORENTZEN May 2013 PUBLISHED BY THE DEFENSE ACQUISITION UNIVERSITY PRESS PROJECT ADVISOR: BOB SKERTIC CAPITAL AND NORTHEAST REGION, DAU THE SENIOR SERVICE COLLEGE FELLOWSHIP PROGRAM ABERDEEN PROVING GROUND, MD PAGE LEFT BLANK INTENTIONALLY .ARMY ACQUISITION WORKFORCE DEPENDENCY ON E-MAIL FOR FORMAL WORK COORDINATION: FINDINGS AND OPPORTUNITIES FOR WORKFORCE PERFORMANCE IMPROVEMENT THROUGH E-MAIL-BASED SOCIAL NETWORK ANALYSIS KENNETH A. LORENTZEN May 2013 PUBLISHED BY THE DEFENSE ACQUISITION UNIVERSITY PRESS PROJECT ADVISOR: BOB SKERTIC CAPITAL AND NORTHEAST REGION, DAU THE SENIOR SERVICE COLLEGE FELLOWSHIP PROGRAM ABERDEEN PROVING GROUND, MD PAGE LEFT BLANK INTENTIONALLY ii Table of Contents Table of Contents ............................................................................................................................ ii List of Figures ................................................................................................................................ vi Abstract ......................................................................................................................................... vii Chapter 1—Introduction ................................................................................................................. 1 Background and Motivation ................................................................................................. -

Inviwo — a Visualization System with Usage Abstraction Levels
IEEE TRANSACTIONS ON VISUALIZATION AND COMPUTER GRAPHICS, VOL X, NO. Y, MAY 2019 1 Inviwo — A Visualization System with Usage Abstraction Levels Daniel Jonsson,¨ Peter Steneteg, Erik Sunden,´ Rickard Englund, Sathish Kottravel, Martin Falk, Member, IEEE, Anders Ynnerman, Ingrid Hotz, and Timo Ropinski Member, IEEE, Abstract—The complexity of today’s visualization applications demands specific visualization systems tailored for the development of these applications. Frequently, such systems utilize levels of abstraction to improve the application development process, for instance by providing a data flow network editor. Unfortunately, these abstractions result in several issues, which need to be circumvented through an abstraction-centered system design. Often, a high level of abstraction hides low level details, which makes it difficult to directly access the underlying computing platform, which would be important to achieve an optimal performance. Therefore, we propose a layer structure developed for modern and sustainable visualization systems allowing developers to interact with all contained abstraction levels. We refer to this interaction capabilities as usage abstraction levels, since we target application developers with various levels of experience. We formulate the requirements for such a system, derive the desired architecture, and present how the concepts have been exemplary realized within the Inviwo visualization system. Furthermore, we address several specific challenges that arise during the realization of such a layered architecture, such as communication between different computing platforms, performance centered encapsulation, as well as layer-independent development by supporting cross layer documentation and debugging capabilities. Index Terms—Visualization systems, data visualization, visual analytics, data analysis, computer graphics, image processing. F 1 INTRODUCTION The field of visualization is maturing, and a shift can be employing different layers of abstraction. -

Image Processing on Optimal Volume Sampling Lattices
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1314 Image processing on optimal volume sampling lattices Thinking outside the box ELISABETH SCHOLD LINNÉR ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-554-9406-3 UPPSALA urn:nbn:se:uu:diva-265340 2015 Dissertation presented at Uppsala University to be publicly examined in Pol2447, Informationsteknologiskt centrum (ITC), Lägerhyddsvägen 2, hus 2, Uppsala, Friday, 18 December 2015 at 10:00 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Professor Alexandre Falcão (Institute of Computing, University of Campinas, Brazil). Abstract Schold Linnér, E. 2015. Image processing on optimal volume sampling lattices. Thinking outside the box. (Bildbehandling på optimala samplingsgitter. Att tänka utanför ramen). Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1314. 98 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9406-3. This thesis summarizes a series of studies of how image quality is affected by the choice of sampling pattern in 3D. Our comparison includes the Cartesian cubic (CC) lattice, the body- centered cubic (BCC) lattice, and the face-centered cubic (FCC) lattice. Our studies of the lattice Brillouin zones of lattices of equal density show that, while the CC lattice is suitable for functions with elongated spectra, the FCC lattice offers the least variation in resolution with respect to direction. The BCC lattice, however, offers the highest global cutoff frequency. The difference in behavior between the BCC and FCC lattices is negligible for a natural spectrum. We also present a study of pre-aliasing errors on anisotropic versions of the CC, BCC, and FCC sampling lattices, revealing that the optimal choice of sampling lattice is highly dependent on lattice orientation and anisotropy. -

IUS 2020 Table of Contents
IUS 2020 Table of Contents IUS 2020 Table of Contents .................................................................................................................................1 IUS 2020 Patrons & Sponsor ..............................................................................................................................2 IUS 2020 Short Courses .......................................................................................................................................5 IUS 2020 Opening/Closing Events ....................................................................................................................7 IUS 2020 Live Social/Workshop Events ...........................................................................................................7 Technical Program Table of Contents .......................................................................................................... 14 1 IUS 2020 Patrons & Sponsor Platinum Patron Verasonics designs and markets leading-edge Vantage™ ultrasound research systems for academic and commercial investigators. These real-time, software-based, programmable ultrasound systems accelerate research by providing unsurpassed speed and control to simplify the data collection and analysis process. Researchers in 34 countries routinely use the unparalleled flexibility of the Vantage platform to advance the art and science of ultrasound through their own research efforts. In addition, to protect your investment and encompass additional research options, every Vantage -

Dissecting the Fornix in Basic Memory Processes and 12 13 Neuropsychiatric Disease: a Review 14 Susan L
Manuscript 1 2 3 4 5 6 7 8 9 10 11 Dissecting the fornix in basic memory processes and 12 13 neuropsychiatric disease: A review 14 Susan L. Benear, Chi T. Ngo & Ingrid R. Olson 15 Temple University, Philadelphia, PA 19122 16 17 18 19 20 21 Address correspondence to: 22 23 Ingrid R. Olson E-mail: [email protected] 24 Department of Psychology T: (215) 204-7318 25 Temple University F: (215) 204- 5539 26 th 27 1701 N. 13 Street 28 Philadelphia, PA 19122 29 30 31 32 Number of words in abstract: 169 33 Number of tables, figures: 3 tables and 4 figures 34 35 36 Keywords: fornix, white matter, episodic memory, hippocampus, diffusion tensor imaging, 37 Alzheimer’s Disease, epilepsy, acetylcholine, theta, obesity, nucleus accumbens, deep brain 38 stimulation, schizophrenia, stress, bipolar disorder, mood 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 1 2 3 4 Abstract 5 6 The fornix is the primary axonal tract of the hippocampus, connecting it to modulatory 7 subcortical structures. This review reveals that fornix damage causes cognitive deficits that 8 closely mirror those resulting from hippocampal lesions. In rodents and non-human primates, 9 this is demonstrated by deficits in conditioning, reversal learning, and navigation. In humans, 10 this manifests as anterograde amnesia. The fornix is essential for memory formation because it 11 12 serves as the conduit for theta rhythms and acetylcholine, as well as providing mnemonic 13 representations to deep brain structures that guide motivated behavior, such as when and where 14 to eat. -

An Afferent Hippocampal Fiber System in the Fornix of the Monkey
An Afferent Hippocampal Fiber System in the Fornix of the Monkey CHARLES L. VOTAW AND EDWARD W. LAUER Department of Anatmy, The University of Michigan, Ann ATboT, Michigan In a previous investigation (Votaw, ’60b) and weighed from 2.2 to 5.3 kgs. Seven it was noted that, in spite of massive bilat- were used only for this investigation and eral removal of the hippocampal formation seven others were used as well in another (Ammon’s horn and dentate gyms), there investigation on the function of the hippo- was a considerable percentage of the fi- campus and are included in this study bers in the fornix, anterior to the hippo- because the lesions placed were ideally carnpal commissure, that did not degener- suited for comparison with the former ate. This would imply, since the lesions seven cases. to all intents and purposes eliminated all All animals were subjected to aseptic possible fibers going into the fornix from surgical procedures. The only surgical pro- the region of the temporal lobe, the pres- cedure on seven was the production of the ence of fibers in the fornix which are a€- lesions to be reported here; lesions were ferent with respect to the hippocampus, placed in the remaining seven after other and further, that these fibers are not com- studies had been carried out. General missural in nature. In another study anesthesia was used and consisted of (Votaw, ’60a), it was shown that lesions ether, sodium pentobarbital (20-25 mg/kg in the septa1 area would produce a de- of body weight given intravenously) or a generation which could be followed from combination of the two anesthetics, ether the area of the lesion in a caudal direction, being used to supplement a somewhat coursing through the body of the fornix, lower dose of sodium pentobarbital. -

Medline-Sourced Journals Are Indicated in Green) Print-ISSN E-ISSN
Sourcerecord id Source Title (Medline-sourced journals are indicated in Green) Print-ISSN E-ISSN 18500162600 21st Century Music 15343219 21100404576 2D Materials 20531583 21100447128 3 Biotech 2190572X 21905738 21100779062 3D Printing and Additive Manufacturing 23297662 23297670 21100229836 3D Research 20926731 19700200922 3L: Language, Linguistics, Literature 01285157 145295 4OR 16194500 16142411 16400154734 A + U-Architecture and Urbanism 03899160 5700161051 A Contrario 16607880 21100399164 A&A case reports 23257237 21100881366 A&A practice 25753126 19600162043 A.M.A. American Journal of Diseases of Children 00968994 19400157806 A.M.A. archives of dermatology 00965359 19600162081 A.M.A. Archives of Dermatology and Syphilology 00965979 19400157807 A.M.A. archives of industrial health 05673933 19600162082 A.M.A. Archives of Industrial Hygiene and Occupational Medicine 00966703 19400157808 A.M.A. archives of internal medicine 08882479 19400158171 A.M.A. archives of neurology 03758540 19400157809 A.M.A. archives of neurology and psychiatry 00966886 19400157810 A.M.A. archives of ophthalmology 00966339 19400157811 A.M.A. archives of otolaryngology 00966894 19400157812 A.M.A. archives of pathology 00966711 19400157813 A.M.A. archives of surgery 00966908 21100456161 a/b: Auto/Biography Studies 21517290 11600153683 A|Z ITU Journal of Faculty of Architecture 13028324 21100780699 A+BE Architecture and the Built Environment 22123202 22147233 5800207606 AAA, Arbeiten aus Anglistik und Amerikanistik 01715410 28033 AAC: Augmentative and Alternative -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -
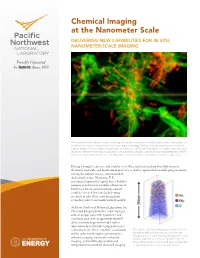
Chemical Imaging at the Nanometer Scale Delivering New Capabilities for in Situ, Nanometer-Scale Imaging
Chemical Imaging at the Nanometer Scale DELIVERING NEW CAPABILITIES FOR IN SITU, NANOMETER-ScALE IMAGING The majority of microbes in natural and engineered environments live within structured communities or biofilms (seen here using confocal laser scanning microscopy). Biofilms include a poorly characterized organic matrix, termed extracellular polymeric substance (EPS), which facilitates certain biogeochemical reactions. We are enhancing visualization, compositional analysis, and functional characterization of EPS to better understand its influence on subsurface reactions vital to environmental and energy issues. Having a complete, precise, and realistic view of the molecular interactions that occur in chemical, materials, and biochemical processes is vital to exponential scientific progress and to solving the nation’s energy, environmental, and security issues. Numerous U.S. government-sponsored reports have identified imaging as critical for scientific advancement. However, current instrumentation cannot reach the needed level of clarity, leaving scientists to infer what is occurring from secondary sources and mathematical models. At Pacific Northwest National Laboratory, the Chemical Imaging Initiative is developing a suite of unique tools with nanometer-scale resolution and element specificity that will allow scientists to go from model system observation to real-world manipulation on a molecular level. These real-time, in situ tools Through the Chemical Imaging Initiative at Pacific will be achieved through near-nanometer Northwest -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons.