Laser Tutorial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aquarius Series Blue Laser Pointer This Blue Laser Pointer Will Captivate Audiences Wherever You Take It, Whether at Home with F
216-5 ADRIAN AVE. TORONTO, ON, CANADA M6N5G4 T. 1.416.729.7976 F. 1.480.247.4864 [email protected] Aquarius Series Blue Laser Pointer This blue laser pointer will captivate audiences wherever you take it, whether at home with friends or at a presentation conference with colleagues. Blue laser pointers are still so rare that few have ever seen one. Be one of the first to own this unique product but don’t blame us if you keep getting asked how you got one! Key Features: • Starting at: $395 • Output Power: Varies with model • Expected Life: 5000 hours • Wavelength: 473nm (blue) • Key Feature: Most technologically advanced portable laser • Package Includes: Portable laser, aluminum carrying case, instructions/warranty. • Casing: Hard anodized machined aluminum • Safety Info: Complies with Class IIIa regulations • Duty Cycle: 90 sec. on/20 sec. off ©2009 LASERGLOW TECHNOLOGIES Page 1 of 3 Item Details Price (USD) Sustained: 0.6-2.0mW Aquarius-2 $395 Peak: ~3mW Sustained: 2-5mW Aquarius-5 $449 Peak: <5mW Sustained: 5-9mW Aquarius-6 $499 Peak: ~15mW Sustained:10-19mW Aquarius-10 $599 Peak: ~25mW Sustained: 20-39mW Aquarius-20 $799 Peak: ~45mW Sustained: 40-59mW Aquarius-40 $1,199 Peak: ~65mW Sustained: 60-79mW Aquarius-60 $1,599 Peak: ~85mW SPECIFICATIONS: Series Name Aquarius Series Dimensions (mm) 155 x 34 Battery Type 1 pcs. lithium 3V "CR-2" battery Sustained Output Power 2-25 mW Wavelength 473 nm Output Type Pulsed (700Hz) Cooling Method Air Beam Color Blue Transverse Mode TEM00, TEM01, TEM02 Beam Diameter <1.0 mm Beam Divergence <1.0 mrad Operating Temperature 20 - 35°C Expected Lifetime 5,000 hours Warranty Period 6 months ©2009 LASERGLOW TECHNOLOGIES Page 2 of 3 If selecting an Aquarius over 5mW, you should be aware that this laser is not FDA compliant and is intended for use in countries that do not require FDA Class IIIb certification for lasers over 5mW. -

Injection Locking of New Focus Lasers for 100+ Mw of Power at 461 Nm
TECHNICAL APPLICATION NOTE Injection Locking of New Focus Lasers for 100+ mW of Power at 461 nm 1. Introduction The GaN blue light-emitting diode, first demonstrated by Akasaki, Amano, and independently by Nakamura earned them the Nobel Prize in Physics in 2014 and has revolutionized modern lighting technology. This led to many new developments, one of which was the blue laser diode which has similarly transformed the way researchers obtain laser light at blue wavelengths. The blue laser diode has also greatly reduced the cost and level of complexity involved with such experiments. The blue laser diode can be integrated into an external cavity with wavelength-selective optics to make a tunable external cavity diode laser (ECDL). One example of this is the New Focus TLB-6802 Vortex™ Plus tunable Figure 1. Fluorescence emitted by strontium atoms (blue dot in center of laser which adopts a Littman-Metcalf configuration to give continuously viewport) trapped in a MOT. Number density is estimated at 1 0 11 cm -3 . tunable output in the blue in a single longitudinal mode and boasts an output Image courtesy of Francisco Camarga, Tom Killian Lab, Rice University. power greater than 40 mW. And in the world of atomic physics, several In injection locking of lasers, a small fraction of the output of a “master” laser neutral atoms and ions of alkaline earth metals have electronic transitions in is injected into the gain region of a free-running, higher-powered “slave” the blue and can therefore be studied using the Vortex Plus. For example, laser. By doing so, the slave laser inherits many of the optical properties of neutral strontium’s 1S -1P main cooling transition is resonant with 461 nm 0 1 master laser including single-mode output, narrow linewidth, and frequency light. -

Opacity Corrections for Resonance Silver Lines in Nano-Material Laser-Induced Plasma
atoms Article Opacity Corrections for Resonance Silver Lines in Nano-Material Laser-Induced Plasma Ashraf M. EL Sherbini 1, Ahmed H. EL Farash 2, Tharwat M. EL Sherbini 1 and Christian G. Parigger 3,* 1 Laboratory of Laser and New Materials, Faculty of Science, Cairo University, Giza 12613, Egypt 2 Engineering Physics Department, Military Technical College, Cairo 11766, Egypt 3 Department of Physics and Astronomy, University of Tennessee, University of Tennessee Space Institute, Center for Laser Applications, 411 B. H. Goethert Parkway, Tullahoma, TN 37388, USA * Correspondence: [email protected]; Tel.: +1-931-841-5690 Received: 21 June 2019; Accepted: 29 July 2019; Published: 31 July 2019 Abstract: Q-switched laser radiation at wavelengths of 355, 532, and 1064 nm from a Nd: YAG laser was used to generate plasma in laboratory air at the target surface made of nano-silver particles of size 95 10 nm. The emitted resonance spectra from the neutral silver at wavelengths of 327.9 nm ± and 338.2 nm indicate existence of self-reversal in addition to plasma self-absorption. Both lines were identified in emission spectra at different laser irradiation wavelengths with characteristic dips at the un-shifted central wavelengths. These dips are usually associated with self-reversal. Under similar conditions, plasmas at the corresponding bulk silver target were generated. The recorded emission spectra were compared to those obtained from the nano-material target. The comparisons confirm existence of self-reversal of resonance lines that emerge from plasmas produced at nano-material targets. This work suggests a method for recovery of the spectral line shapes and discusses practical examples. -

Sirolaser Blue— Three Wavelenghts with One Single Device
I industry SIROLaser Blue— Three wavelenghts with one single device Author_Marlene Hartinger, Germany Fig. 1 Fig. 1_All participants of the _Dental diode lasers _Three wavelengths—one device 2nd Sirona Laser Days. The use of laser in dentistry has steadily grown over At this year’s IDS in Cologne, Sirona introduced the past decades as lasers have repeatedly proven to be SIROLaser Blue, the first dental diode laser with a blue, powerful surgical tools for both hard and soft tissue ap- infrared and red diode. By providing three wavelengths plications. There is no discipline in dentistry that does (445 nm, 970 nm, 660 nm) with one single device, not benefit from the advantages of laser therapy. SIROLaser Blue enables a spectrum of 21 indications in- Among dental lasers currently available, diode lasers cluding frenectomy, fibroma, gingivoplasty, tissue have become particularly popular due to their compact management and haemostasis. The blue laser light at a size, versatility and relatively affordable pricing. Diode wavelength of 445 nm is used in soft-tissue surgery be- lasers use a semiconductor stimulated by electricity to cause it is absorbed more effectively by tissue com- produce laser light and enable practitioners to perform pared to infrared laser light. Due to its shorter wave- less invasive procedures with greater patient comfort. length, it does not penetrate deeply in surgery and has Swelling, scaring and post-operative pain is consider- consequently less effect on surrounding tissue. The ably minimised and wounds and tissue heal faster. In ad- blue laser makes it possible to work in a non-contact dition, dental diode lasers effectively reduce the level of mode, achieving substantially better cutting results at oral germs and bacteria. -

FABRICATION of TRANSPARENT CERAMIC LASER MEDIA for HIGH ENERGY LASER APPLICATIONS Karn Serivalsatit Clemson University, [email protected]
Clemson University TigerPrints All Dissertations Dissertations 5-2010 FABRICATION OF TRANSPARENT CERAMIC LASER MEDIA FOR HIGH ENERGY LASER APPLICATIONS Karn Serivalsatit Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Part of the Materials Science and Engineering Commons Recommended Citation Serivalsatit, Karn, "FABRICATION OF TRANSPARENT CERAMIC LASER MEDIA FOR HIGH ENERGY LASER APPLICATIONS" (2010). All Dissertations. 525. https://tigerprints.clemson.edu/all_dissertations/525 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. FABRICATION OF TRANSPARENT CERAMIC LASER MEDIA FOR HIGH ENERGY LASER APPLICATIONS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Materials Science and Engineering by Karn Serivalsatit May 2010 Accepted by: Dr. John Ballato, Committee Chair Dr. Stephen Foulger Dr. Jian Luo Dr. Eric Skaar i ABSTRACT Sesquioxides of yttrium, scandium, and lutetium, i.e., Y2O3, Sc 2O3, and Lu 2O3, have received a great deal of recent attention as potential high power solid state laser hosts. These oxides are receptive to lanthanide doping, including trivalent Er, Ho and Tm which have well known emissions at eye-safe wavelengths that can be excited using commercial diode lasers. These sesquioxides are considered superior to the more conventional yttrium aluminum garnet (YAG) due to their higher thermal conductivity, which is critical for high power laser system. Unfortunately, these oxides possess high melting temperatures, which make the growth of high purity and quality crystals using melt techniques difficult. -

Construction of a Flashlamp-Pumped Dye Laser and an Acousto-Optic
; UNITED STATES APARTMENT OF COMMERCE oUBLICATION NBS TECHNICAL NOTE 603 / v \ f ''ttis oi Construction of a Flashlamp-Pumped Dye Laser U.S. EPARTMENT OF COMMERCE and an Acousto-Optic Modulator National Bureau of for Mode-Locking Iandards — NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measure- ment system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scien- tific community, industry, and commerce. The Institute consists of a Center for Radia- tion Research, an Office of Measurement Services and the following divisions: Applied Mathematics—Electricity—Heat—Mechanics—Optical Physics—Linac Radiation 2—Nuclear Radiation 2—Applied Radiation 2—Quantum Electronics 3— Electromagnetics 3—Time and Frequency 3 —Laboratory Astrophysics3—Cryo- 3 genics . -

S. Nakamura, G. Fasol . the Blue Laser Diode
S. Nakamura, G. Fasol . The Blue Laser Diode Springer-Verlag Berlin Heidelberg GmbH Shuji Nakamura Gerhard Fasol The Blue Laser Diode GaN Based Light Emitters and Lasers With 246 Figures and 49 Tables Springer Shuji Nakamura Gerhard Fasol, Ph. D. Nichia Chemical Industries Ltd. Eurotechnology Japan Ltd. 491, Oka, Kaminaka Parkwest Building 11th Floor Anan, Tokushima-ken 774 6-12-1 Nishi-Shinjuku Japan Shinjuku-ku, Tokyo 160 e-mail: [email protected] Japan e-mail: [email protected] Libary of Congress, Cataloging· in-Publication Data applied for Die Deutsche Bibliothek - CIP-Einheitsaufnahme Nakamura, Shuji: The blue laser diode: GaN based light emitters and lasers / Shuji Nakamura; Gerhard Fasol. - Berlin; Heidelberg; New York; Barcelona; Budapest; Hong Kong; London; Milan; Paris; Santa Clara; Singapore; Tokyo: Springer, 1997. NE: Fasol, Gerhard ISBN 978-3-662-03464-4 ISBN 978-3-662-03462-0 (eBook) DOI 10.1007/978-3-662-03462-0 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broad casting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable for prosecution under the German Copyright Law. © Springer-Verlag Berlin Heidelberg 1997 Originally published by Springer-Verlag Berlin Heidelberg New York in 1997. Softcover reprint of the hardcover I st edition 1997 The use of general descriptive names, registered names, trademarks, etc. -
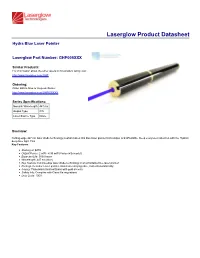
View Spec Sheet
Laserglow Product Datasheet Hydra Blue Laser Pointer Laserglow Part Number: GHP005XXX Similar Products: For information about the other lasers in this product family visit: http://www.laserglow.com/GHP Ordering: Order Online Now or Request Quote: http://www.laserglow.com/GHP005XXX Series Specifications: Nominal Wavelength 447 nm Output Type CW Laser Source Type Diode Overview: Cutting-edge 447 nm laser diode technology is what makes this blue laser pointer both unique and affordable. Keep everyone's attention with the Hydra's deep blue light. This Key Features: Starting at: $279 Output Power: 2 mW - 4.99 mW (Varies with model) Expected Life: 5000 hours Wavelength: 447 nm (blue) Key Feature: Cutting-edge laser diode technology in an affordable blue laser pointer! Package Includes: Laser pointer, aluminum carrying case, instructions/warranty. Casing: Piano-black finished Brass with gold accents Safety Info: Complies with Class IIIa regulations Duty Cycle: 100% Specifications: This spec sheet has been generated specifically for part number GHP005XXX, per your request, and data for the entire series is also displayed for your reference. The specs which are specific to GHP005XXX have been highlighted below in red + bold. Laser Form Factor GAP Output Power (mW) <5 FDA Safety Class IIIa Central Wavelength (nm) 447 Wavelength Tolerance (+/- nm) 5 Divergence (mrad, full angle) <1.5 Beam Dimensions (mm, 1/e²) 5 Transverse Mode Near TEM00 Longitudinal Modes Multiple Approximate Peak Power (W) 5 Operating Temperature Range (°C) -5 to 40 Storage Temperature Range (°C) -10 to 50 Total Power Consumption (W) 1 Max. Power Input Duty Cycle 100% Cooling Method Passive Air Standard Warranty (months) 6 MTTF (operational hours) 5000 Weight of Product or Laser Head (kg) 0.1 Dimensions of Product or Laser Head (mm) 150 (l) x 16.5 (d) Power Supply 2 x CR2 Batteries CW: All specifications are based on performance at full output power and after the specified warmup period. -

Tunable, Low-Repetition-Rate, Cost-Efficient Femtosecond Ti:Sapphire Laser for Nonlinear Microscopy
Appl Phys B (2012) 107:17–22 DOI 10.1007/s00340-011-4830-7 Tunable, low-repetition-rate, cost-efficient femtosecond Ti:sapphire laser for nonlinear microscopy P.G. Antal · R. Szipocs˝ Received: 29 July 2011 / Revised version: 26 September 2011 / Published online: 25 November 2011 © Springer-Verlag 2011 Abstract We report on a broadly tunable, long-cavity light intensity sufficient for nonlinearities, ultrashort (fs or Ti:sapphire laser oscillator being mode-locked in the net ps) pulse mode-locked lasers are used as light sources, most negative intracavity dispersion regime by Kerr-lens mode- often femtosecond pulse, tunable Ti:sapphire lasers having locking, delivering τFWHM < 300 fs pulses at 22 MHz rep- a repetition rate at around 80 MHz. etition rate. The wavelength of the laser can be tuned over Multiphoton transitions of intracellular fluorophores used a 170 nm wide range between 712 nm and 882 nm. Hav- in microscopy can be efficiently excited in the 700–1200 nm ing a typical pump power of 2.6 W, the maximum pulse spectral region, which wavelengths penetrate deeper in tis- peak power is 60 kW. Comparison of the reported laser sues and are much less harmful for living specimen than with a standard, 76 MHz Ti:sapphire oscillator regarding direct UV illumination in single-photon fluorescent mi- two-photon excitation efficiency in a laser scanning micro- croscopy. Photochemical damage mechanisms, which can scope shows that the 22 MHz laser generates the same flu- cause oxidative stress or direct DNA damage, only occur orescence signal at considerably, 1.82 times lower average in the focus of the objective lens, where multiphoton ab- power, which is expected to result in a reduced photothermal sorption takes place. -

Fibre Lasers – Conditioning Constructional and Technological
BULLETIN OF THE POLISH ACADEMY OF SCIENCES OPTOELECTRONICS TECHNICAL SCIENCES, Vol. 58, No. 4, 2010 DOI: 10.2478/v10175-010-0048-9 Fibre lasers – conditioning constructional and technological A. ZAJĄC1,2∗, D. DOROSZ2, M. KOCHANOWICZ2, M. SKÓRCZAKOWSKI1, and J. ŚWIDERSKI1 1, Institute of Optoelectronics, Military University of Technology, 2 Kaliskiego St., 00-908 Warszawa, Poland 2 Faculty of Electrical Engineering, Bialystok University of Technology, 45d Wiejska St., 15-351 Białystok, Poland Abstract. In this paper the actual level of fiber lasers’ development is presented. There is also presented the analysis of technological and constructional conditions that limit energy parameters of those sources. Authors also show a construction and a technological work, conducted in Poland, which led to improving energy and exploit parameters of fiber lasers. Key words: fibre lasers, conditioning constructional, conditioning technological. 1. Introduction Typical, basically known the 1964’s configuration of a fiber laser set-up fulfils two basic conditions: an active cen- The year of 1960 was a breakthrough in the optical science – tre is a core of an optical fiber with addition of rare earth ions on 16th May the first laser was built. (it is likely to use a single-mode fiber but in many applica- The properties of laser beam – monochromatic, coherent tions also a multi-mode fiber is used), an active addition is and collimated beam – had not been possible to get in exist- optically stimulated – for lasers of medium and high power ing sources of optical radiation in visible spectrum until that it is effective in the configuration of lateral pumping (dis- moment. -

Infrared Laser Catheter Apparatus
~" ' MM II II II Ml II I II III II II II I II J European Patent Office © Publication number: 0 214 712 B1 Office_„. europeen- desj brevets^ » © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 02.09.92 © Int. CI.5: A61 B 17/00, A61B 17/36 © Application number: 86303982.2 @ Date of filing: 27.05.86 © Infrared laser catheter apparatus. ® Priority: 31.07.85 US 761188 © Proprietor: C.R. BARD, INC. 731 Central Avenue @ Date of publication of application: Murray Hill New Jersey 07974(US) 18.03.87 Bulletin 87/12 @ Inventor: Slnofsky, Edward © Publication of the grant of the patent: Apartment 8 South Washington Street 02.09.92 Bulletin 92/36 Reading Massachusetts(US) © Designated Contracting States: DE FR GB IT NL © Representative: Woodward, John Calvin et al VENNER SHIPLEY & CO. 368 City Road © References cited: London EC1V 2QA(GB) EP-A- 0 153 847 GB-A- 2 017 506 GB-A- 2 125 986 US-A- 3 327 712 US-A- 4 383 729 US-A- 4 458 683 ELECTRONIC DESIGN, vol. 17, no. 13, June 21, 1969, pp 36, 38, 40 D.N. KAYE: "The happy merger of fiber optics and lasers" 00 CM s O Note: Within nine months from the publication of the mention of the grant of the European patent, any person ^ may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition qj shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid (Art. -

Construction and Measurement of an Actively Mode-Locked Sigma Laser
Calhoun: The NPS Institutional Archive Theses and Dissertations Thesis Collection 1998-06 Construction and measurement of an actively mode-locked sigma laser Butler, James M. Monterey, California. Naval Postgraduate School http://hdl.handle.net/10945/8146 DUOU=\ c SCHOOL <> NPS-EC-98-011 NAVAL POSTGRADUATE SCHOOL MONTEREY, CALIFORNIA THESIS CONSTRUCTION AND MEASUREMENT OF AN ACTIVELY MODE-LOCKED SIGMA LASER by James M. Butler June, 1998 Thesis Co-Advisors: Phillip E. Pace John P. Powers Approved for public release; distribution is unlimited. Prepared for: Center for Reconnaissance Research Naval Postgraduate School Monterey, CA NAVAL POSTGRADUATE SCHOOL Monterey, California 93943 Rear Admiral Chaplain Superintendent This thesis was prepared in conjunction with research sponsored in part by the Center for Reconnaissance Research at the Naval Postgraduate School. Reproduction of all or part of this report is authorized. 1 REPORT DOCUMENTATION PAGE Form Approved OMB N'o 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instruction, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to Washington Headquarters Services, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington DC 20503. 1 . AGENCY USE ONLY (Leave blank) REPORT DATE 3. REPORT TYPE AND DATES COVERED June 1998 Master's Thesis TITLE AND SUBTITLE TITLE.