Guidance for Transporting Ammonia by Rail 2007 Guidance for Transporting Ammonia by Rail
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cooperative Research in Tank Car Safety Design
00_TRN_286_TRN_286 7/11/13 5:04 PM Page 12 Testing of tank car thermal protection at the Transportation Technology Center near Pueblo, Colorado, in the early 1970s helped quantify the rate of heat transfer into a tank under intense fire conditions. Railroads and Research Sharing Track Cooperative Research in Tank Car Safety Design How Science and Engineering Are Reducing the Risk of Rail Transport of Hazardous Materials CHRISTOPHER P. L. BARKAN, M. RAPIK SAAT, FRANCISCO GONZÁLEZ, III, AND TODD T. TREICHEL ailroad tank car safety in North America has improved continuously through Barkan is Professor, Department of Civil and cooperative testing, research, and standards development by industry and Environmental Engineering, and Executive government. Although much of this progress has been evolutionary, in recent Director, Rail Transportation and Engineering R decades more revolutionary approaches have taken hold. Center, University of Illinois at Urbana– The railroad, tank car, and petrochemical industries have worked together with Champaign. Saat is Research Assistant the government to develop and improve safety design standards for tank cars since Professor, University of Illinois at Urbana– the early 20th century (1). In 1903, the Master Car Builders’ Association formed the Champaign. González is Hazardous Materials Committee on Tank Cars, composed of the mechanical officers from several railroads and Tank Car Project Manager, Office of and a representative from Union Tank Line, then the major tank car owner. The com- Research and Development, Federal Railroad mittee recommended practices that were soon established as industry standards for Administration, Washington, D.C. Treichel is the construction and repair of tank cars. Director, Railway Supply Institute–Association The American Railway Association and its successor, the Association of American of American Railroads Railroad Tank Car Safety TR NEWS 286 MAY–JUNE 2013 TR NEWS 286 MAY–JUNE Railroads (AAR), later adopted the standards. -
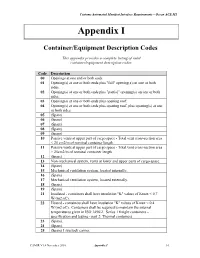
Appendix I – Container/Equipment Description Codes
Customs Automated Manifest Interface Requirements – Ocean ACE M1 Appendix I Container/Equipment Description Codes This appendix provides a complete listing of valid container/equipment description codes. Code Description 00 Openings at one end or both ends. 01 Opening(s) at one or both ends plus "full" opening(s) on one or both sides. 02 Opening(s) at one or both ends plus "partial" opening(s) on one or both sides. 03 Opening(s) at one or both ends plus opening roof. 04 Opening(s) at one or both ends plus opening roof, plus opening(s) at one or both sides. 05 (Spare) 06 (Spare) 07 (Spare) 08 (Spare) 09 (Spare) 10 Passive vents at upper part of cargo space - Total vent cross-section area < 25 cm2/m of nominal container length. 11 Passive vents at upper part of cargo space - Total vent cross-section area > 25cm2/m of nominal container length. 12 (Spare) 13 Non-mechanical system, vents at lower and upper parts of cargo space. 14 (Spare) 15 Mechanical ventilation system, located internally. 16 (Spare) 17 Mechanical ventilation system, located externally. 18 (Spare) 19 (Spare) 21 Insulated - containers shall have insulation "K" values of Kmax < 0.7 W/(m2.oC). 22 Heated - containers shall have insulation "K" values of Kmax < 0.4 W/(m2.oC). Containers shall be required to maintain the internal temperatures given in ISO 1496/2. Series 1 freight containers – specification and testing - part 2: Thermal containers. 23 (Spare). 24 (Spare). 25 (Spare) Livestock carrier. CAMIR V1.4 November 2010 Appendix I I-1 Customs Automated Manifest Interface Requirements – Ocean ACE M1 Code Description 26 (Spare) Automobile carrier. -

Railroad Emergency Response Manual
Metropolitan Washington Council of Governments Railroad Emergency Response Manual Approved by the COG Fire Chiefs Committee Metropolitan Washington Council of Governments Second Edition May 2020 MWCOG Railroad Emergency Response Manual 2nd Edition – May 2020 ACKNOWLEDGEMENTS This manual could not have been written without the assistance of many Dedicated rail safety personnel and members of the Metropolitan Washington Council of Governments regional emergency response agencies that have spent many hours providing the material for the creation of this manual. We thank all emergency responders from all jurisdictions, including our federal agency partners that shared their firsthand experiences of recent commuter railroad incidents. Many of their experiences were incorporated into sections of this manual. Many Railroad representatives, private industry and governmental organizations provided their invaluable technical assistance. This committee would like to thank Steve Truchman formerly of the National Railroad Passenger Corporation (Amtrak), Greg Deibler from Virginia Railway Express (VRE), David Ricker from the Maryland Rail Commuter (MARC), Paul Williams of Norfolk Southern Railway Corporation and Mike Hennessey of CSX Transportation, all of whom provided the specific diagrams, illustrations and other technical information regarding railroad equipment. We recognize Elisa Nichols of Kensington Consulting, LLC for her contributions to this manual as well as representatives from many Federal Agencies who also provided information on the technical accounts of railroad equipment and their integrity on past railroad incidents. The members of the Metropolitan Washington Council of Governments (COG) Passenger Rail Safety Subcommittee gratefully presents this manual to both Fire and Rescue Service and Railroad organizations in an effort to instill readiness within our own personnel that they might effectively and collaboratively respond to a railroad incident. -

Stifel 2016 Transportation & Logistics Conference
GATX Corporation Stifel 2016 Transportation & Logistics Conference Unless otherwise noted, GATX is the source for data provided NYSE: GMT Forward-Looking Statements Forward-looking statements in this press release that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These include statements that reflect our current views with respect to, among other things, future events, financial performance and market conditions. In some cases, forward-looking statements can be identified by the use of words such as “may,” “could,” “expect,” “intend,” “plan,” “seek,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “likely,” “will,” “would,” and variations of these terms and similar expressions, or the negative of these terms or similar expressions. Specific risks and uncertainties include, but are not limited to, (1) the impact of new regulatory requirements for tank cars carrying crude, ethanol, and other flammable liquids; (2) inability to maintain our assets on lease at satisfactory rates; (3) weak economic conditions, financial market volatility, and other factors that may decrease demand for our assets and services; (4) decreased demand for railcars due to sustained low crude oil prices; (5) reduced opportunities to generate asset remarketing income; (6) changes to, or failure to comply with, laws, rules, and regulations applicable to our assets and operations; (7) operational disruption and increased costs associated with compliance maintenance -

Proper Car Placement in Trains
Proper Car Placement in Trains Presented by DuPage Division Midwest Region NMRA . Presented at Badgerland Express Madison, Wisconsin JJam Introduction Some of you may wonder why it is important to know that cars can be placed in the wrong order in a train. And, why does this even matter on a model railroad? On a real railroad improper car placement can cause embarrassing situations such as this .............. (Picture a train wreck.) Proper car placement also insures the general safety of.... The merchandise being transported The safety of the train crews handling the cars and the train The safety of the community thru which these cars may move. Some of these rules can be utilized to make the operation of your models more prototypical. The last reason I can think of for knowing this valuable information is that you can use it to heckle another modeler about his operation. ("Do you know that that tank car is in the wrong place in that train?") How do you know how to properly place cars in a train? There are a whole bunch of rulebooks. charts and other documents that trainmen must be familiar with when switching and operating trains. Every railroad has a "Book of Rules" which is the basis of this information. Superceding the "Book of Rules" is the Time Table and Special Instructions which is specific to a certain portion of the railroad. This is superceded by the System and General Bulletins. The most specific information is found in the Train Messages which are issued with every work order. Here is the hierarchy triangle: SYSTEM AND GENERAL BULLETINS TIME TABLE AND SPECIAL INSTRUCTIONS GENERAL OPERATING RULES (BOOK) lets start with a few general rules to set the stage: Rule A Employees whose duties are governed by these rule must provide themselves with a copy of these rules... -

The Safe Rail Transport of Fertilizer Food, Feed, Fuel Fiber
The Fertilizer Institute FERTILIZER’S ROLE in AGRICULTURE Nourish, Replenish, Grow The safe rail transport of fertilizer is critical for the production of food, feed, fuel fiber. NITROGEN (N) is a primary building block for all ertilizer major crop nutrients& are nitrogen, phosphorus and potash organisms. It is essential to making – all naturally occurring elements in the environment – which proteins, helps keep plants green and is a critical component of soil F are “fed” to plants and crops for healthy and abundant food structure. production. Crops need nutrients to grow and be productive – just like comes from the air humans. The world’s population is 6.5 billion and growing. Using fertilizers in an agronomically and environmentally sensitive way is critical for our planet’s increasing need for food, feed, fuel and fiber. Ammonia is the most economical and efficient source of nitrogen for most farmers and is the primary ingredient in most all nitrogen fertilizers. PHOSPHORUS (P) Additionally, it is an essential ingredient in many finished phosphate is found in every living cell. Phosphorus is a component of DNA and it also fertilizers. Ammonia is transported by rail, truck, barge or pipeline from plays vital roles in capturing light the production site to a fertilizer terminal or to a fertilizer dealer or farm during photosynthesis, helping with seed germination, and helping plants cooperative for formulation into other fertilizers or direct application in the use water efficiently. Plants also use phosphorus to help fight external stress fields. and prevent disease. comes from ancient sea life POTASSIUM (K) is essential to the workings of every living cell. -

Railroads and Research Railroads and Research
00_TRN_286_TRN_286 7/11/13 5:04 PM Page C1 MAY–JUNE 2013 NUMBER 286 TR NEWS Railroads and Research Sharing Track Ⅲ Impact Tests and Crashworthiness Ⅲ Safety Design for Hazmat Tank Cars Ⅲ Track Support for Increased Volumes Ⅲ Buying-In to Safety Culture Ⅲ Reducing Grade Crossing Incidents Ⅲ Implementing Positive Train Control Ⅲ Aligning Research Approaches 00_TRN_286_TRN_286 7/11/13 5:04 PM Page C2 TRANSPORTATION RESEARCH BOARD 2013 EXECUTIVE COMMITTEE* Chair: Deborah H. Butler, Executive Vice President, Planning, and CIO, Norfolk Southern Corporation, Norfolk, Virginia National Academy of Sciences Vice Chair: Kirk T. Steudle, Director, Michigan Department of Transportation, Lansing National Academy of Engineering Executive Director: Robert E. Skinner, Jr., Transportation Research Board Institute of Medicine National Research Council Victoria A. Arroyo, Executive Director, Georgetown Climate Center, and Visiting Professor, Georgetown University Law Center, Washington, D.C. The Transportation Research Board is one Scott E. Bennett, Director, Arkansas State Highway and Transportation Department, Little Rock of six major divisions of the National William A. V. Clark, Professor of Geography (emeritus) and Professor of Statistics (emeritus), Department of Geography, University of California, Los Angeles Research Council, which serves as an James M. Crites, Executive Vice President of Operations, Dallas–Fort Worth International Airport, Texas independent adviser to the federal gov- Malcolm Dougherty, Director, California Department of Transportation, -

Rolling Stock: Locomotives and Rail Cars
Rolling Stock: Locomotives and Rail Cars Industry & Trade Summary Office of Industries Publication ITS-08 March 2011 Control No. 2011001 UNITED STATES INTERNATIONAL TRADE COMMISSION Karen Laney Acting Director of Operations Michael Anderson Acting Director, Office of Industries This report was principally prepared by: Peder Andersen, Office of Industries [email protected] With supporting assistance from: Monica Reed, Office of Industries Wanda Tolson, Office of Industries Under the direction of: Deborah McNay, Acting Chief Advanced Technology and Machinery Division Cover photo: Courtesy of BNSF Railway Co. Address all communication to Secretary to the Commission United States International Trade Commission Washington, DC 20436 www.usitc.gov Preface The United States International Trade Commission (USITC) has initiated its current Industry and Trade Summary series of reports to provide information on the rapidly evolving trade and competitive situation of the thousands of products imported into and exported from the United States. Over the past 20 years, U.S. international trade in goods and services has risen by almost 350 percent, compared to an increase of 180 percent in the U.S. gross domestic product (GDP), before falling sharply in late 2008 and 2009 due to the economic downturn. During the same two decades, international supply chains have become more global and competition has increased. Each Industry and Trade Summary addresses a different commodity or industry and contains information on trends in consumption, production, and trade, as well as an analysis of factors affecting industry trends and competitiveness in domestic and foreign markets. This report on the railway rolling stock industry primarily covers the period from 2004 to 2009, and includes data for 2010 where available. -

Do's & Don'ts Poster for General Service Cars
DO’S Do’s and Dont’s of General Service Tank Car Handling IT IS the Shipper’S reSponSibilitY to bottom unloading. This is essentially a plug type valve mounted to nar.aar.com To ensure safe operating procedures, ENSURE THAT NO a saddle on the bottom of the tank that is controlled at the top of commoDitY IS leAKinG follow safety rules, practice proper the tank via a rod attachment. When the valve cover is removed, From ANY component it may be inverted and used as a tool to operate the valve. maintenance and use common sense. OR THE TANK ITSELF DO - verify car stencilling information such as car number, BEFORE SHIPPING A CAR. specification, HM201 qualification, dates, tank, contents, etc. The vapor valve is typically a ball type valve. It is usually attached 1 2 General service tank cars are equipped to the top of the fittings coverplate using flanged or screwed connections. Since it does not extend into the liquid, it will only with various top and bottom fittings to expel vapor when opened. 3 Do’s AND Don’ts allow loading, unloading, gauging and 4 testing operations. For additional During a top unloading process, the unloading line is attached to 5 OF GENERAL SERVICE the liquid valve, and an air or gas line is attached to the vapor 6 information, read AAR BOE Pamphlet 34, valve. Pressure is applied to the tank by forcing air or an inert gas 8 DO - vent the interior tank pressure DO - use the stuffing box cover TaNK CAR HANDLING before loosening manway cover or a square drive tool to operate the into the vapor space of the tank through the vapor valve such that bolts and hold down before loading/ top operated bottom outlet valve rod. -

Transportation Vessels
Transportation Vessels MC-306 (DOT-406) Non (low) pressure bulk liquid cargo tank • MC 306--Maximum operating pressure of up to 3 psi • DOT 406--Maximum operating pressure of up to 4 psi • Oval or “egg” shaped cross section • Flat or nearly flat ends • Aluminum—primary material of construction • Usually multi-compartmented • Separate manhole for each compartment • Emergency shutoff –drivers side front • Rollover protection to prevent manholes from opening up on rollover • Average maximum capacity--9, 000 gallons Contents: • NORMALLY—petroleum products, however, may be water or milk MC-307 (DOT-407) Low-pressure bulk liquid cargo tank • Pressure—up to 40 psi @ 70ºF • Horse shaped” shaped cross section • Flat or slightly rounded ends • Stainless steel is primary material of construction • May be insulated. Insulation may hide tank shape—may not appear to be horseshoe shaped. • Manway usually center (top) of tank • Discharge valves usually center (bottom) of tank • If multi-compartmented, will have a separate manhole for each compartment • Emergency shutoff –drivers side front • Rollover protection to prevent manholes from opening up on rollover, normally center of tank top • Average maximum capacity--6, 000 gallons Contents: • NORMALLY—mild acids, however, may be water, milk, or combustible liquids MC-312 (DOT-412) Corrosives Cargo Tank • Pressure—5 to 25 psiExterior strengthening (stiffener) rings often visible • Lined tank—usually lined with rubber or plastic • Round cross section • Stainless steel is primary material of construction -

Best Practices for Rail Transport of Fuel Ethanol
BEST PRACTICES FOR RAIL TRANSPORT OF FUEL ETHANOL This document was prepared by the Renewable Fuels Association (RFA). The information, though believed to be accurate at the time of publication, should not be considered as legal advice or as a substitute for developing specific company operating guidelines. The RFA does not make any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or applicability of the information presented in this document. www.ethanolrfa.org/producers/safety September 2017 Best Practices for Rail Transport of Fuel Ethanol Table of Contents The Fuel Ethanol Marketplace ...................................................................................................................... 2 Railcar 101; Video Link .................................................................................................................................. 4 Training Requirements .................................................................................................................................. 5 Regulatory Compliance, Fines and Penalties ................................................................................................ 6 DOT Registration Requirement ..................................................................................................................... 7 Continuous Improvement, Non-Accidental Releases (NAR) ......................................................................... 8 New DOT-117 Rail Tank Car ......................................................................................................................... -

Caboose Progress the Freighthouse So It Could Accept Visitors
Issue Number 57 San Luis Obispo, California Fall 2016 www.slorrm.com Museum open every Saturday 10:00 to 4:00; other times for groups by arrangement. Contact [email protected]. Recognition for Major Supporters A new board mounted in the exhibit hall identifies Last in the Train – But First On the List those who made major contributions to upgrading Caboose Progress the Freighthouse so it could accept visitors. Volunteers have given high priority to restoring form- er Southern Pacific bay window caboose #1886 –seats, flooring, painting, hardware, and fixtures—aiming for public tours by the Railroad Festival in October. Work has progressed with the support of several Museum and community members. Brad LaRose, Dennis Lynch, Dan Manion, and Bob Wilson in particular have spent many hours returning the heavily used and somewhat abused interior to near original condition. Cabooses accommodated freight conductors and brakemen, who worked with waybills and switch lists, monitored brake-line air pressure, kept an eye on the whole train, and protected the rear of the train. The body of a bay window caboose was narrower than a typical car, with the bay’s slanted end windows allowing a clear view of the train on even gradual curves. SP used bay window cabooses from the late 1940s through the end of the Tank Car Arrives at the Museum “caboose era” in the 1980s. Three photos by Glen Matteson Several years of searching, negotiating, and planning paid off July 29 when this former Southern Pacific tank car arrived at the Museum. Thanks to Curator Brad LaRose’s diligence, it was donated by Union Pacific Railroad.