States of Matter Project Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monday Morning, October 19, 2015
Monday Morning, October 19, 2015 Energy Frontiers Focus Topic Willow glass is a new material introduced recently to the market, while nickel is a inexpensive flexible reflective foil. The Corning Willow glass is Room: 211B - Session EN+AS+EM+NS+SE+SS+TF- coated with a molybdenum layer as a reflective back contact layer. By using MoM a single step and a solution deposition method, lower production cost are achievable. For thin film deposition, we used a non-vacuum spin coater (WS650 spin processor, Laurell Technologies) with an optimized spin coat Solar Cells I programming. Annealing took place under vacuum in a RTP furnace while Moderator: Jason Baxter, Drexel University, Chintalapalle time, temperature and ramp functions were varied. The other layers of the Ramana, University of Texas at El Paso device consists of cadmium sulfide n-type window layer and a zinc oxide doped with aluminum transparent top contact layer. Characterization and analysis of the thin films were performed using Raman spectroscopy, 8:20am EN+AS+EM+NS+SE+SS+TF-MoM1 Elevated Temperature scanning election microscope (Zeiss NEON 40), X-ray diffraction (Philipps Phase Stability of CZTS-Se Thin Films for Solar Cells, E. Chagarov, K. X’Pert), proflimeter (Veeco Dektak 150), UV-Vis-NIR Spectrophotometer Sardashti, University of California at San Diego, D.B. Mitzi, Duke (Carry 5000), Hall Effect measurement system (HMS3000) and 4 point University, R.A. Haight, IBM T.J. Watson Research Center, Andrew C. probe (Lucas Labs) measurements. Results show CZTS thin film solar cells Kummel, University of California at San Diego on flexible glass is obtainable. -

JOURNAL of CONDENSED MATTER NUCLEAR SCIENCE Experiments and Methods in Cold Fusion
JOURNAL OF CONDENSED MATTER NUCLEAR SCIENCE Experiments and Methods in Cold Fusion Proceedings of the first French Symposium RNBE-2016 on Condensed Matter Nuclear Science (Réactions Nucléaires à Basse Énergie), March 18–20, 2016 VOLUME 21, November 2016 JOURNAL OF CONDENSED MATTER NUCLEAR SCIENCE Experiments and Methods in Cold Fusion Editor-in-Chief Jean-Paul Biberian Marseille, France Editorial Board Peter Hagelstein Xing Zhong Li Edmund Storms MIT, USA Tsinghua University, China KivaLabs, LLC, USA George Miley Michael McKubre Fusion Studies Laboratory, SRI International, USA University of Illinois, USA JOURNAL OF CONDENSED MATTER NUCLEAR SCIENCE Volume 21, November 2016 © 2016 ISCMNS. All rights reserved. ISSN 2227-3123 This journal and the individual contributions contained in it are protected under copyright by ISCMNS and the following terms and conditions apply. Electronic usage or storage of data JCMNS is an open-access scientific journal and no special permissions or fees are required to download for personal non-commercial use or for teaching purposes in an educational institution. All other uses including printing, copying, distribution require the written consent of ISCMNS. Permission of the ISCMNS and payment of a fee are required for photocopying, including multiple or systematic copying, copying for advertising or promotional purposes, resale, and all forms of document delivery. Permissions may be sought directly from ISCMNS, E-mail: [email protected]. For further details you may also visit our web site: http:/www.iscmns.org/CMNS/ Members of ISCMNS may reproduce the table of contents or prepare lists of articles for internal circulation within their institutions. Orders, claims, author inquiries and journal inquiries Please contact the Editor in Chief, [email protected] or [email protected] J. -
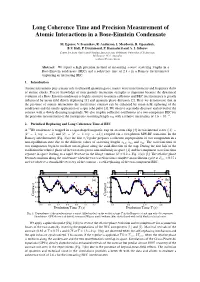
Long Coherence Time and Precision Measurement of Atomic Interactions in a Bose-Einstein Condensate
Long Coherence Time and Precision Measurement of Atomic Interactions in a Bose-Einstein Condensate M. Egorov, V. Ivannikov, R. Anderson, I. Mordovin, B. Opanchuk, B.V. Hall, P. Drummond, P. Hannaford and A. I. Sidorov Centre for Atom Optics and Ultrafast Spectroscopy, Swinburne University of Technology, Melbourne 3122, Australia [email protected] Abstract: We report a high precision method of measuring s-wave scattering lengths in a Bose-Einstein condensate (BEC) and a coherence time of 2.8 s in a Ramsey interferometer employing an interacting BEC. 1. Introduction Atomic interactions play a major role in ultracold quantum gases, matter-wave interferometry and frequency shifts of atomic clocks. Precise knowledge of inter-particle interaction strengths is important because the dynamical evolution of a Bose-Einstein condensate is highly sensitive to atomic collisions and BEC interferometry is greatly influenced by mean-field driven dephasing [1] and quantum phase diffusion [2]. Here we demonstrate that in the presence of atomic interactions the interference contrast can be enhanced by mean-field rephasing of the condensate and the timely application of a spin echo pulse [3]. We observe a periodic decrease and revival of the contrast with a slowly decaying magnitude. We also employ collective oscillations of a two-component BEC for −4 the precision measurement of the interspecies scattering length a12 with a relative uncertainty of 1.6 × 10 . 2. Periodical Rephasing and Long Coherence Time of BEC A 87Rb condensate is trapped in a cigar-shaped magnetic trap on an atom chip [4] in two internal states (|1 = |F =1,mF = −1 and |2 = |F =2,mF =+1) coupled via a two-photon MW-RF transition. -

Research Article Nuclear Processes in Dark Interstellar Matter of H(0) Decrease the Hope of Migrating to Exoplanets
AAAS Space: Science & Technology Volume 2021, Article ID 9846852, 6 pages https://doi.org/10.34133/2021/9846852 Research Article Nuclear Processes in Dark Interstellar Matter of H(0) Decrease the Hope of Migrating to Exoplanets Leif Holmlid Department of Chemistry and Molecular Biology, University of Gothenburg, SE-412 96 Göteborg, Sweden Correspondence should be addressed to Leif Holmlid; [email protected] Received 24 November 2020; Accepted 11 August 2021; Published 3 September 2021 Copyright © 2021 Leif Holmlid. Exclusive Licensee Beijing Institute of Technology Press. Distributed under a Creative Commons Attribution License (CC BY 4.0). It is still generally assumed that interstellar travel will be possible after purely technical development and thus that mankind can move to some suitable exoplanet when needed. However, recent research indicates this not to be the case, since interstellar space is filled with enough ultradense hydrogen H(0) as stable condensed dark matter (Holmlid, Astrophysical Journal 2018) to make interstellar space travel at the required and technically feasible relativistic velocities (Holmlid et al, Acta Astronautica 2020) almost impossible. H(0) can be observed to exist in space from the so-called extended red emission (ERE) features observed in space. A recent review (Holmlid et al., Physica Scripta 2019) describes the properties of H(0). H(0) gives nuclear processes emitting kaons and other particles, with kinetic energies even above 100 MeV after induction for example by fast particle (spaceship) impact. These high particle energies give radiative temperatures of 12000 K in collisions against a solid surface and will rapidly destroy any spaceship structure moving into the H(0) clouds at relativistic velocity. -

GOTHIC Michelucci Alessandro All Is Light Intelligent Design GOTHIC - All Is Light - Intelligent Design
GOTHIC Michelucci Alessandro All is Light Intelligent Design GOTHIC GOTHIC - All is light - intelligent design - intelligent - All is light Alessandro Michelucci EOTI This work is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecom- mons.org/licenses/by-sa/3.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA Publication: EOTI Composition and typesetting: EOTI Published in Berlin - Germany ISBN 978-3-00-061246-6 EOTI - European Organization of Translators and Interpreters® Internet presence: www.eoti.eu e-mail: [email protected] EOTI - Heidenfeldstraße 8 10249 - Berlin “The reasons for using the Garamond typefaces rather than Gothic typefaces do not lie in the Antiqua-Fraktur dispute, but rather in the desire to recall the French Renaissance form.” “Sigillum burgensium de berlin sum” (Ich bin das Siegel der Bürger von Berlin) GOTHIC All is Light Intelligent Design Written by Alessandro Michelucci EOTI vi INDEX PREFACE ............................................................................................................ viii I. LIGHT AS “THEOLOGICAL SYMBOL”.................................................................. 1 II. LIGHT AS “CONCEPT OF MERE VISION”........................................................ 13 III. ALL IS LIGHT ............................................................................................... 24 IV. A NEW POLITICAL VISION ........................................................................... -

Redshifts in Space Caused by Stimulated Raman Scattering in Cold Intergalactic Rydberg Matter with Experimental Verification¦ L
Journal of Experimental and Theoretical Physics, Vol. 100, No. 4, 2005, pp. 637–644. From Zhurnal Éksperimental’noÏ i TeoreticheskoÏ Fiziki, Vol. 127, No. 4, 2005, pp. 723–731. Original English Text Copyright © 2005 by Holmlid. NUCLEI, PARTICLES, FIELDS, GRAVITATION, AND ASTROPHYSICS Redshifts in Space Caused by Stimulated Raman Scattering in Cold Intergalactic Rydberg Matter with Experimental Verification¶ L. Holmlid Atmospheric Science, Department of Chemistry, Göteborg University, SE-412 96 Göteborg, Sweden e-mail: [email protected] Received September 23, 2004 Abstract—The quantized redshifts observed from galaxies in the local supercluster have recently been shown to be well described by stimulated Stokes Raman processes in intergalactic Rydberg matter (RM). The size of the quanta corresponds to transitions in the planar clusters forming the RM, of the order of 6 × 10–6 cm–1. A stimulated Stokes Raman process gives redshifts that are independent of the wavelength of the radiation, and it allows the radiation to proceed without deflection, in agreement with observation. Such redshifts must also be additive during the passage through space. Rydberg matter is common in space and explains the observed Fara- day rotation in intergalactic space and the spectroscopic signatures called unidentified infrared bands (UIBs) and diffuse interstellar bands (DIBs). Rydberg matter was also recently proposed to be baryonic dark matter. Experi- ments now show directly that IR light is redshifted by a Stokes stimulated Raman process in cold RM. Shifts of 0.02 cm–1 are regularly observed. It is shown by detailed calculations based on the experimental results that the redshifts due to Stokes scattering are of at least the same magnitude as observations. -

Impurity Recombination of Rydberg Matter E
Impurity recombination of Rydberg matter E. A. Manykin, M. I. Ozhovan, and P. P. Polu6ktov Russian Scientific Center "Kurchatov Institute," 123182 Moscow, Russia (Submitted 25 June 1993) Zh. Eksp. Teor. Fiz. 105, 50-61 (January 1994) We consider the trapping of electrons by electronegative impurities from the conduction band for condensed systems of highly excited atoms (Rydberg matter). The trapping can occur both with photon emission and through a nonradiative mechanism. In both cases the transition of electrons from the conduction band of the condensate to impurity orbitals is possible in practice only in the regions where the electron motion is classically admissible. We show that the rate of radiative processes increases with an increase in the quantum- mechanical level of excitation of the atoms, whereas the rate of the nonradiative processes decreases rapidly. When the impurity density is comparable to the skeleton density of the Rydberg matter, the trapping of electrons by impurities leads to a fast decay of the condensate. At the same time, for low densities of the impurities, after electron trapping they will be incorporated into the structure of the Rydberg matter; as a result, the recombination of excitations is improbable. 1. INTRODUCTION equilibrium parameters of RM in the framework of a den- sity functional theory using the concept of the pseudopo- One can invoke condensed excited states (CES) to de- tential of excited atoms from which the CES is formed.' scribe dense systems of excited centers (atoms, molecules, For definiteness, we give briefly the numerical parameters impurities in solids).'.' The well known electron-hole of RM formed as the result of the condensation of cesium state, which occurs as the result of the condensation of atoms excited in an s state. -

Coulombic Phase Transitions in Dense Plasmas
Coulombic Phase Transitions in Dense Plasmas∗ W. Ebeling1, G. Norman2 1 Institut f¨ur Physik, Humboldt-Universit¨at Berlin, Invalidenstrasse 110, D-10115 Berlin, Germany; [email protected] 2 Institute for High Energy Densities, AIHT of RAS, Izhorskaya street 13/19, Moscow 125412, Russia; henry [email protected] We give a survey on the predictions of Coulombic phase transitions in dense plasmas (PPT) and derive several new results on the properties of these transitions. In particular we discuss several types of the critical point and the spinodal curves of quantum Coulombic systems. We construct a simple theoretical model which shows (in dependence on the parameter values) either one alkali- type transition (Coulombic and van der Waals forces determine the critical point) or one Coulombic transition and another van der Waals transition. We investigate the conditions to find separate Van der Waals and Coulomb transitions in one system (typical for hydrogen and noble gas-type plasmas). The separated Coulombic transitions which are strongly influenced by quantum effects are the hypothetical PPT, they are in full analogy to the known Coulombic transitions in classical ionic systems. Finally we give a discussion of several numerical and experimental results referring to the PPT in high pressure plasmas. I. FIRST ORDER VAN DER WAALS AND range repulsion. We remember that the two phases in COULOMB TRANSITIONS the van der Waals model differ from each other by the density of molecules. The two phases in PPT have dif- ferent number densities of charged particles and different The theory of gases developed in the dissertation of degrees of ionization. -

Editorial for Whichever Issue of Molecular Physics Includes TMPH 219660, the Paper by Professor Leif Holmlid
Editorial for whichever issue of Molecular Physics includes TMPH 219660, the paper by Professor Leif Holmlid. Tim Softley To cite this version: Tim Softley. Editorial for whichever issue of Molecular Physics includes TMPH 219660, the pa- per by Professor Leif Holmlid.. Molecular Physics, Taylor & Francis, 2007, 105 (08), pp.923-924. 10.1080/00268970701428568. hal-00513109 HAL Id: hal-00513109 https://hal.archives-ouvertes.fr/hal-00513109 Submitted on 1 Sep 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Molecular Physics For Peer Review Only Editorial for whichever issue of Molecular Physics includes TMPH 219660, the paper by Professor Leif Holmlid. Journal: Molecular Physics Manuscript ID: TMPH-2007-0104 Manuscript Type: Preliminary Communication Date Submitted by the 19-Apr-2007 Author: Complete List of Authors: Softley, Tim; University of Oxford Keywords: Editorial URL: http://mc.manuscriptcentral.com/tandf/tmph Page 1 of 3 Molecular Physics 1 2 3 Editorial: Rydberg Matter 4 5 6 In this issue we publish a paper by L. Holmlid which reports the observation of 7 radiofrequency emission spectra that are attributed to rotational transitions of planar 8 Rydberg matter clusters K*19 . -

Proposal for the Development of a Compact Neutron Source, Bonp1183r3
Proposal for the development of a compact neutron source Victor de Haan, BonPhysics BV Dag Zeiner-Gundersen, Norrønt AS Sindre Zeiner-Gundersen, Norrønt AS © 2019, BonPhysics BV, BONP1183r3 Tel: +31(0)78 6767023 Email: [email protected] Website: www.bonphysics.nl Handelsregisternummer 23085607 van de Kamer van Koophandel Rotterdam IBAN: NL28KNAB0255336187 BIC: KNABNL2H BTW/VAT: NL805662571B01 Proposal for the development of a compact neutron source, BONP1183r3 Preface This proposal gives an overview of the investment needed for the realization of proof of principle and further development of a compact neutron source based on the research into Rydberg Matter by Prof. L. Holmlid and his collaborators. The energy technology based on this research is being developed by Norrønt AS. The research into Rydberg Matter by Prof. L. Holmlid has resulted in a method to produce neutrons via muon-catalyzed fusion of hydrogen isotopes. The projected optimized intensity of this neutron source is between 10 14 to 10 16 neutrons per second which is 2 to 4 orders of magnitude larger than state-of-the-art neutron generators. This neutron intensity in a relatively small volume is comparable to research reactor sources based on fission of uranium, but the amount of radiation and radioactive waste produced will be negligible compared to fission sources. This makes this source a perfect candidate for the replacement of these research reactors in the future where each neutron spectrometer can have its own neutron source. The contents of this proposal was generated by BonPhysics by means of resources of BonPhysics BV, resources of Norrønt AS, references as quoted in the reference list. -

Rydberg Matter in Space: Low-Density Condensed Dark Matter
Mon. Not. R. Astron. Soc. 333, 360–364 (2002) Rydberg matter in space: low-density condensed dark matter Shahriar Badiei and Leif HolmlidP Reaction Dynamics Group, Department of Chemistry, Go¨teborg University, SE-412 96 Go¨teborg, Sweden Accepted 2002 February 4. Received 2002 January 10; in original form 2001 August 10 Downloaded from https://academic.oup.com/mnras/article/333/2/360/1019164 by guest on 28 September 2021 ABSTRACT Here Rydberg matter is proposed as a candidate for the missing dark matter or dark baryonic matter in the Universe. Spectroscopic and other experimental studies give valuable informa- tion on the properties of Rydberg matter, especially its very weak interaction with light caused by the very small overlap with low states, and because of the necessary two-electron transitions even for disturbed matter. Recently, the unidentified infrared (UIR) bands have been shown to agree well with calculations and experiments on Rydberg matter. This is the reason for the present, somewhat speculative, proposal that dark matter has, at least partially, the form of Rydberg matter. The UIR bands have also been observed directly in emission from Rydberg matter in the laboratory. The unique space-filling properties of Rydberg matter are described: a hydrogen atom in this matter occupies a volume 5 £ 1012 times larger than in its ground state or in a hydrogen molecule. Key words: ISM: general – ISM: molecules – dark matter. observations and theory. Another interesting proposal concerning 1 INTRODUCTION the baryon part of dark matter is the existence of cold dense clouds Dark matter is a kind of matter that accounts for the missing mass, of hydrogen molecules (Lequex, Allen & Guilloteau 1993; that is the mass that is non-luminous but must exist in the galaxies Kalberla, Kerp & Haud 2000). -

And Deuterium D(0) and Their Relation to Ordinary Rydberg Matter: a Review
Physica Scripta PAPER • OPEN ACCESS Ultradense protium p(0) and deuterium D(0) and their relation to ordinary Rydberg matter: a review To cite this article: Leif Holmlid and Sindre Zeiner-Gundersen 2019 Phys. Scr. 94 075005 View the article online for updates and enhancements. This content was downloaded from IP address 131.169.5.251 on 06/05/2019 at 14:09 Physica Scripta Phys. Scr. 94 (2019) 075005 (26pp) https://doi.org/10.1088/1402-4896/ab1276 Ultradense protium p(0) and deuterium D(0) and their relation to ordinary Rydberg matter: a review Leif Holmlid1 and Sindre Zeiner-Gundersen2 1 Atmospheric Science, Department of Chemistry and Molecular Biology, University of Gothenburg, SE-412 96, Göteborg, Sweden 2 Norrønt AS, Vaterlandsveien 19, 3470 Slemmestad, Norway and Science Institute, University of Iceland, Dunhaga 3, 107 Reykjavik, Iceland E-mail: [email protected] Received 5 November 2018, revised 22 February 2019 Accepted for publication 22 March 2019 Published 24 April 2019 Abstract The extremely large density of ultra-dense hydrogen H(0) has been proved in numerous experiments by three laser-induced methods, namely Coulomb explosions observed by particle time-of-flight (TOF) and TOF mass spectrometry, rotational emission spectroscopy in the visible, and annihilation-like meson ejecting nuclear reaction processes. The density of H(0) at the quite common spin level s = 2 is of the order of 100 kg cm−3. The theory of ultra-dense hydrogen H(0) is described briefly, especially the ‘mixed’ spin quantum number s and its relation to the internuclear distances. The orbital angular momentum of the bonding electrons in H(0) is l=0, which gives the H(0) designation.