Postembryonic Development of the Unique Antenna of Mantophasmatodea (Insecta)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Freshwater Fishes
WESTERN CAPE PROVINCE state oF BIODIVERSITY 2007 TABLE OF CONTENTS Chapter 1 Introduction 2 Chapter 2 Methods 17 Chapter 3 Freshwater fishes 18 Chapter 4 Amphibians 36 Chapter 5 Reptiles 55 Chapter 6 Mammals 75 Chapter 7 Avifauna 89 Chapter 8 Flora & Vegetation 112 Chapter 9 Land and Protected Areas 139 Chapter 10 Status of River Health 159 Cover page photographs by Andrew Turner (CapeNature), Roger Bills (SAIAB) & Wicus Leeuwner. ISBN 978-0-620-39289-1 SCIENTIFIC SERVICES 2 Western Cape Province State of Biodiversity 2007 CHAPTER 1 INTRODUCTION Andrew Turner [email protected] 1 “We live at a historic moment, a time in which the world’s biological diversity is being rapidly destroyed. The present geological period has more species than any other, yet the current rate of extinction of species is greater now than at any time in the past. Ecosystems and communities are being degraded and destroyed, and species are being driven to extinction. The species that persist are losing genetic variation as the number of individuals in populations shrinks, unique populations and subspecies are destroyed, and remaining populations become increasingly isolated from one another. The cause of this loss of biological diversity at all levels is the range of human activity that alters and destroys natural habitats to suit human needs.” (Primack, 2002). CapeNature launched its State of Biodiversity Programme (SoBP) to assess and monitor the state of biodiversity in the Western Cape in 1999. This programme delivered its first report in 2002 and these reports are updated every five years. The current report (2007) reports on the changes to the state of vertebrate biodiversity and land under conservation usage. -

Cederberg-IDP May 2020 – Review 2020-2021
THIRD REVIEW: 2020/2021 MAY 2020 SECTIONS REVISED THIRD REVISION TO THE FOURTH GENERATION IDP ................... 0 3.8. INTERGOVERNMENTAL RELATIONS ................................. 67 FOREWORD BY THE EXECUTIVE MAYOR.................................. 2 3.9. INFORMATION AND COMMUNICATION TECHNOLOGY (ICT) ...... 68 ACKNOWLEDGEMENT FROM THE MUNICIPAL MANAGER AND IMPORTANT MESSAGE ABOUT COVID-19 ................................. 4 CHAPTER 4: STRATEGIC OBJECTIVES AND PROJECT ALIGNMENT .. 71 EXECUTIVE SUMMARY ....................................................... 5 4.1 IMPROVE AND SUSTAIN BASIC SERVICE DELIVERY AND CHAPTER I: STATEMENT OF INTENT ...................................... 9 INFRASTRUCTURE .................................................... 73 1.1. INTRODUCTION ......................................................... 9 A. Water B. Electricity 1.2. THE FOURTH (4TH) GENERATION IDP .............................. 10 C. Sanitation D. Refuse removal / waste management 1.3. THE IDP AND AREA PLANS ........................................... 11 E. Roads F. Comprehensive Integrated Municipal Infrastructure Plan 1.4. POLICY AND LEGISLATIVE CONTEXT ................................ 11 G. Stormwater H. Integrated Infrastructure Asset Management Plan 1.5. STRATEGIC FRAMEWORK OF THE IDP .............................. 13 I. Municipal Infrastructure Growth Plan 1.6. VISION, MISSION, VALUES ............................................ 14 4.2 FINANCIAL VIABILITY AND ECONOMICALLY SUSTAINABILITY .... 87 1.7. STRATEGIC OBJECTIVES ............................................ -

Western Cape Biodiversity Spatial Plan Handbook 2017
WESTERN CAPE BIODIVERSITY SPATIAL PLAN HANDBOOK Drafted by: CapeNature Scientific Services Land Use Team Jonkershoek, Stellenbosch 2017 Editor: Ruida Pool-Stanvliet Contributing Authors: Alana Duffell-Canham, Genevieve Pence, Rhett Smart i Western Cape Biodiversity Spatial Plan Handbook 2017 Citation: Pool-Stanvliet, R., Duffell-Canham, A., Pence, G. & Smart, R. 2017. The Western Cape Biodiversity Spatial Plan Handbook. Stellenbosch: CapeNature. ACKNOWLEDGEMENTS The compilation of the Biodiversity Spatial Plan and Handbook has been a collective effort of the Scientific Services Section of CapeNature. We acknowledge the assistance of Benjamin Walton, Colin Fordham, Jeanne Gouws, Antoinette Veldtman, Martine Jordaan, Andrew Turner, Coral Birss, Alexis Olds, Kevin Shaw and Garth Mortimer. CapeNature’s Conservation Planning Scientist, Genevieve Pence, is thanked for conducting the spatial analyses and compiling the Biodiversity Spatial Plan Map datasets, with assistance from Scientific Service’s GIS Team members: Therese Forsyth, Cher-Lynn Petersen, Riki de Villiers, and Sheila Henning. Invaluable assistance was also provided by Jason Pretorius at the Department of Environmental Affairs and Development Planning, and Andrew Skowno and Leslie Powrie at the South African National Biodiversity Institute. Patricia Holmes and Amalia Pugnalin at the City of Cape Town are thanked for advice regarding the inclusion of the BioNet. We are very grateful to the South African National Biodiversity Institute for providing funding support through the GEF5 Programme towards layout and printing costs of the Handbook. We would like to acknowledge the Mpumalanga Biodiversity Sector Plan Steering Committee, specifically Mervyn Lotter, for granting permission to use the Mpumalanga Biodiversity Sector Plan Handbook as a blueprint for the Western Cape Biodiversity Spatial Plan Handbook. -

Downloaded from Their Website
University of Cape Town Faculty of Science Department of Botany THESIS Submitted in fulfilment of the requirements for the degree of Master of Philosophy (Botany) January 2009 An environmental history of the Cederberg: changing climate, land use and vegetation patterns Daniela Vincenza Bonora Supervisors: Prof. Timm Hoffman (Botany, UCT), Dr. Rick Rohde (Centre of African Studies, University of Edinburgh) and Dr. Edmund February (Botany, UCT) ii PLAGIARISM DECLARATION 1. I know that plagiarism is wrong. Plagiarism is to use another’s work and pretend that it is my own. 2. Each contribution to, and quotation in this research report from the work(s) of other people has been contributed, and has been cited and referenced. 3. This research report is my own work and has not previously in its entirety or in part been submitted at any other university for another degree. 4. I have not allowed, and will not allow, anyone to copy my work with the intention of passing it off as his or her own work. Signature: Date: iii An environmental history of the Cederberg: changing climate, land use and vegetation patterns Daniela Vincenza Bonora BNRDAN001 THESIS Submitted in fulfilment of the requirements for the degree of Master of Philosophy (Botany) January 2009 iv - TABLE OF CONTENTS - ACKNOWLEDGEMENTS ................................................................................................................. v ABSTRACT ......................................................................................................................................... -

Flower Route Map 2017
K o n k i e p en w R31 Lö Narubis Vredeshoop Gawachub R360 Grünau Karasburg Rosh Pinah R360 Ariamsvlei R32 e N14 ng Ora N10 Upington N10 IAi-IAis/Richtersveld Transfrontier Park Augrabies N14 e g Keimoes Kuboes n a Oranjemund r Flower Hotlines O H a ib R359 Holgat Kakamas Alexander Bay Nababeep N14 Nature Reserve R358 Groblershoop N8 N8 Or a For up-to-date information on where to see the Vioolsdrif nge H R27 VIEWING TIPS best owers, please call: Eksteenfontein a r t e b e e Namakwa +27 (0)72 760 6019 N7 i s Pella t Lekkersing t Brak u Weskus +27 (0)63 724 6203 o N10 Pofadder S R383 R383 Aggeneys Flower Hour i R382 Kenhardt To view the owers at their best, choose the hottest Steinkopf R363 Port Nolloth N14 Marydale time of the day, which is from 11h00 to 15h00. It’s the s in extended ower power hour. Respect the ower Tu McDougall’s Bay paradise: Walk with care and don’t trample plants R358 unnecessarily. Please don’t pick any buds, bulbs or N10 specimens, nor disturb any sensitive dune areas. Concordia R361 R355 Nababeep Okiep DISTANCE TABLE Prieska Goegap Nature Reserve Sun Run fels Molyneux Buf R355 Springbok R27 The owers always face the sun. Try and drive towards Nature Reserve Grootmis R355 the sun to enjoy nature’s dazzling display. When viewing Kleinzee Naries i R357 i owers on foot, stand with the sun behind your back. R361 Copperton Certain owers don’t open when it’s overcast. -

Cultural Transformation Process
CULTURAL TRANSFORMATION PROCESS • This process sets out to review existing policies on arts, culture and heritage presently utilised by the Department of Cultural Affairs and Sport of the Western Cape Provincial Government. • The national White Paper on Arts, Culture and Heritage has been utilised by the Department as the guide to practice. • After the dawn of our new democracy in 1994 we need to reassess our progress and learn from the lessons of the past years. This policy provides a new vision and policy direction for the Department and its statutory bodies. It was decided at a Western Cape Provincial Cabinet Lekgotla that the MEC for Cultural Affairs, Sport and Recreation, should host a Cultural Transformation Indaba during August 2004 for most of the stakeholders in the arts, culture and heritage sphere in the Western Cape. The purpose of the indaba was to provide a platform for interactive debate in order to kick-start the development of transformed policies that aid efficient and effective service delivery in the arts, culture and heritage field in line with the vision of Cabinet to “make the Western Cape a home for all”. At the indaba the way forward was divided into various phases: • A consolidated report on the indaba would be provided by the facilitators. • The deliberations at the indaba would be taken into account by the Department in the development of its strategic plans. • A reference group would be appointed by the Department to assist in the drafting of the policy. • The draft policy itself. • A Cultural Impact Conference would be held at the Cape Town Convention Centre. -
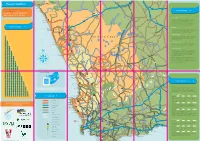
Flower Route Map 2015 FA
K o n k i e p en w R31 Lö Narubis Vredeshoop Gawachub R360 Grünau Karasburg Rosh Pinah R360 Ariamsvlei R32 e N14 ng Ora N10 Upington N10 IAi-IAis/Richtersveld Transfrontier Park Augrabies N14 e g Keimoes Kuboes n a Oranjemund r Flower Hotlines H O a ib R359 Holgat Kakamas Alexander Bay Nababeep N14 Nature Reserve R358 Groblershoop N8 N8 O r a For up-to-date information on where to see the Vioolsdrif nge H R27 VIEWING TIPS best owers, please call: Eksteenfontein a r t e b e e Namakwa +27 (0)79 294 7260 N7 st Pella i Lekkersing t Brak u West Coast +27 (0)72 938 8186 o N10 Pofadder S R383 R383 Aggeneys Flower Hour i R382 Kenhardt To view the owers at their best, choose the hottest Steinkopf R363 Port Nolloth N14 Marydale time of the day, which is from 11h00 to 15h00. It’s the s in extended ower power hour. Respect the ower Tu McDougall’s Bay paradise: Walk with care and don’t trample plants unnecessarily. Please don’t pick any buds, bulbs or R358 N10 specimens, nor disturb any sensitive dune areas. Concordia R361 R355 Nababeep Okiep DISTANCE TABLE Prieska Goegap Nature Reserve Sun Run els Molyneux Bu R355 Springbok R27 The owers always face the sun. Try and drive towards Nature Reserve Grootmis Kleinzee Naries i R355 the sun to enjoy nature’s dazzling display. When viewing i R357 owers on foot, stand with the sun behind your back. R361 Copperton Certain owers don’t open when it’s overcast. -

Cederberg Municipality Draft Idp (2012-2017)
CEDERBERG MUNICIPALITY DRAFT IDP (2012-2017) 1 TABLE OF CONTENTS CHAPTER ONE .............................................................................................................................................................................................. 6 BACKGROUND AND PROCESS ................................................................................................................................................................ 6 1.1 BACKGROUND ....................................................................................................................................................................................................................................................................................... 6 1.2 PROCESS FOLLOWED IN THE DEVELOPMENT OF THE DRAFT IDP .......................................................................................................................................................................................... 8 A. Preparation Phase........................................................................................................................................................................................................................................................................ 8 A.2 Scoping the Draft IDP process.............................................................................................................................................................................................................................................. 8 -

Parliament of the Province of the Western Cape
Thursday, 24 August 2017] 185 No 51 - 2017] FOURTH SESSION, FIFTH PARLIAMENT PARLIAMENT OF THE PROVINCE OF THE WESTERN CAPE ========================== ANNOUNCEMENTS, TABLINGS AND COMMITTEE REPORTS ========================== THURSDAY, 24 AUGUST 2017 COMMITTEE REPORT Participating Cluster B committees and composition of delegation The following Committees took part in the visit week: Standing Committee on Economic Opportunities, Tourism and Agriculture; Standing Committee on Environmental Affairs and Development Planning; Standing Committee on Finance; Standing Committee on Human Settlements; Standing Committee on Local Government; Standing Committee on Transport and Public Works. The Delegation consisted of the following members: Beerwinkel, CF (ANC); Davids, SW (ANC); Dijana, TM (ANC); Dugmore, CM (ANC); Hinana, NE (DA Co-ordinating Chairperson and leader of the delegation); Joseph, BD (EFF); Joseph, D (DA); Maseko, LM (DA); Mnqasela, M (DA); Nkondlo, ND (ANC); Schäfer, BA (DA); Tyatyam, SG (ANC) ; Uys, P (ANC). The following staff attended the visit week: Adams, Z (Committee Co-ordinator); Barends, A (Driver: Security and Facilities); Coetzee, J (Committee Co-ordinator); Jones, S (Committee Co-ordinator); Niekerk, S (Committee Co-ordinator); and Vermeulen, J (Senior Committee Co-ordinator). 1. Introduction The Joint Visit Week is a key mechanism established by the Western Cape Provincial Parliament to achieve its Constitutional oversight mandate within the province. The visit 186 week is intended to ensure that members of the Provincial Parliament, through its standing committees, continuously keep abreast with developments and service delivery issues in the province. It also affords the opportunity to undertake oversight visits and interact with communities and institutions in order to assess the needs and challenges they experience. -

Section 1 Introduction
Saldanha Development Zone Pre-Feasibility Analysis – Final Report _OCTOBER 2009 SECTION 1 INTRODUCTION 1.1 PURPOSE OF STUDY Demacon Market Studies , Bigen Africa Services and Plan Associates were commissioned by WESGRO (on behalf of the Department of Economic Development and Tourism ) to undertake the task of conducting an Economic and Technical Pre-feasibility Study for an Industrial Development Zone (IDZ) within the proposed Saldanha Industrial Area (i.e. “corridor” as indicated by the SDF ). The pre-feasibility study will investigate the potential to stimulate further industrial and business activities as well as to link these and current activities to the benefits of the proposed Saldanha Development Zone. The Saldanha Development Zone could be seen as the missing link in connecting the various industries and harbours of the surrounding areas. This process will encompass many different disciplines such as economic analysis, technical analysis, spatial planning etc. This process is about unleashing the industrial potential of the Saldanha Bay Municipal area in order to recommend the way forward for an IDZ and the implementation thereof within the area. The objective of this report is to assess the viability of establishing an IDZ at the port of Saldanha and to develop an independent, objective specialist report detailing the anticipated economic and technical net benefits (and disbenefits) of the proposed IDZ and ancillary business activities to the region, province and national economy. 1.2 STUDY AREA DELINEATION Situated approximately 120 km’s north-west of Cape Town, with its natural deep water harbour and its associated development potential, Saldanha has been identified as a Presidential Development Growth Node. -

Polygalaceae)
S.AfrJ.Bot., 1991, 57(5): 229-233 229 A re-evaluation of Nylandtia (Polygalaceae) C.T. Johnson* and F.M. Weitz Department of Botany, University of the Western Cape, Private Bag X17, Bellville, 7535 Republic of South Africa Accepted 10 June 1991 A re-evaluation of the monotypic genus Nylandtia Dumort. is presented in which two varieties, N. spinosa (L.) Dumort. var. spinosa and var. scoparia (Eckl. & Zeyh.) Johnson & Weitz, are recognized. Descriptions, synonymy, distribution data, and a key to the varieties are provided. Variation in the two varieties is covered and pollination ecology is discussed. 'n Re-evaluasie van die monotipiese genus Nylandtia Dumort. word aangebied waarin twee varieteite, N. spinosa (L.) Dumort. var. spinosa en var. scoparia (Eckl. & Zeyh.) Johnson & Weitz, erken word. Beskrywings, sinonimie, verspreidingsdata en 'n sleutel tot die varieteite word voorsien. Variasies tussen die twee varieteite word behandel en die bestuiwingsekologie word bespreek. Keywords: Nylandtia, taxonomy, morphology. ·To whom correspondence should be addressed. History and relationships related. Anatomical data (Johnson, Cyster & Spangenberg The first description referring to this species dates back to 1981) such as cyclocytic stomata, cuticle structure and 1751, when Linnaeus published his Amoenitates academi absence of extraxylary fibres, support Levyns (1949) who, cae. An identical description of the species was given by on the basis of floral structure, regarded Nylandtia as a him in 1753 when he published the name Polygala spinosa, specialized off-shoot of the sub-genus Psilocladus of though the collector or supplier of the specimen was not Muraltia. cited. The specimen that is currently housed in the Linnaean The infraspecific taxonomy in Nylandtia has been herbarium was collected by Rijk Tulbagh in May 1769 variously treated. -

FET College Location by Gross Geographic Product
Mpumalanga: FET college location by Gross Geographic Product In Mpumalanga there are 17 FET colleges and college campuses. None is located in the southeast or southwest of the province. All the colleges and college campuses (Nkangala, Gert Sibande and Ehlanzeni) are located within municipalities within the two highest categories of GGP. Dipaleseng municipality has a low GGP, but no colleges are located there. 103 Northern Cape: FET college location by Gross Geographic Product In the Northern Cape there are nine college campuses within two colleges: Northern Cape Urban and Northern Cape Rural. These colleges have a presence in Kimberley, Kuruman, Okiep, De Aar, Upington and Kathu. The colleges in Kimberley are the only ones located in an area of high GGP. The De Aar campus of Northern Cape Rural is located in a municipality with relatively low GGP, and might struggle financially. 104 North West: FET college location by Gross Geographic Product There are 14 college campuses in North West; all of these are located in municipalities in the top two categories of GGP. All the colleges are located on national or main roads and are therefore very accessible. Vusulela FET in Taung is the only one serving the western part of the province. In the east of the province there is a concentration of colleges in the Klerksdorp and Potchefstroom municipalities. 105 Western Cape: FET college location by Gross Geographic Product In the Western Cape, the municipalities with the highest production are the City of Cape Town, Stellenbosch, Drakenstein, Breede Valley, and George. South Cape College has a presence in the latter municipality and also in the surrounding municipalities of Mossel Bay, Oudtshoorn, and Plettenberg Bay.