Why Does the Us Department of Veterans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FALCON V, LLC, Et Al.,1 DEBTORS. CASE NO. 19-10547 CHAPT
Case 19-10547 Doc 103 Filed 05/21/19 Entered 05/21/19 08:56:32 Page 1 of 13 UNITED STATES BANKRUPTCY COURT MIDDLE DISTRICT OF LOUISIANA IN RE: CASE NO. 19-10547 FALCON V, L.L.C., et al.,1 CHAPTER 11 DEBTORS. (JOINTLY ADMINISTERED) ORDER APPROVING FALCON V, L.L.C.'S ACQUISITION OF ANADARKO E&P ONSHORE LLC’S INTEREST IN CERTAIN OIL, GAS AND MINERAL INTERESTS Considering the motion of the debtors-in-possession, Falcon V, L.L.C., (“Falcon”) for an order authorizing Falcon’s acquisition of the interest of Anadarko E&P Onshore LLC (“Anadarko”) in certain oil, gas and mineral leases (P-13), the evidence admitted and argument of counsel at a May 14, 2019 hearing, the record of the case and applicable law, IT IS ORDERED that the Debtors are authorized to take all actions necessary to consummate the March 1, 2019 Partial Assignment of Oil, Gas and Mineral Leases (the “Assignment”) by which Anadarko agreed to assign its right, title and interest in and to certain oil, gas and mineral leases in the Port Hudson Field, including the Letter Agreement between Falcon and Anadarko attached to this order as Exhibit 1. IT IS FURTHERED ORDERED that notwithstanding anything to the contrary in this order, the relief granted in this order and any payment to be made hereunder shall be subject to the terms of this court's orders authorizing debtor-in-possession financing and/or granting the use of cash collateral in these chapter 11 cases (including with respect to any budgets governing or related to such use), and the terms of such financing and/or cash collateral orders shall control if 1 The Debtors and the last four digits of their respective taxpayer identification numbers are Falcon V, L.L.C. -

Coalition Ad 1/4 Advocate
THANK YOU LOUISIANA LEGISLATURE The Coalition for a Tobacco-Free Louisiana would like to thank the leadership of the following legislators for restoring people’s right to breathe clean air in many workplaces and public places. SENATE President John Hainkel Lynn Dean Jon Johnson Craig Romero Robert Adley Reggie Dupre Bill Jones Tom Schedler Diana Bajoie Noble Ellington C. D. Jones Mike Smith Robert Barham Francis Heitmeier Art Lentini Greg Tarver Lambert Boissiere Don Hines Max Malone Gerald Theunissen Joel Chaisson Kip Holden Joe McPherson Jerry Thomas Don Cravins Ken Hollis Mike Michot Chris Ullo Jay Dardenne Paulette Irons Willie Mount HOUSE OF REPRESENTATIVES Speaker Charlie Dewitt Tommy Wright Michael Jackson Roy Quezaire John Alario Hollis Downs Ronnie Johns Cedric Richmond Damon Baldone Sydnie Mae Durand Kay Katz Joe Salter Clara Baudoin Dale Erdey Lelon Kenney Steve Scalise Ernest Baylor James Fannin Eric LaFleur Melinda Schwegmann Gary Beard Rick Farrar Mitch Landrieu Buddy Shaw Sharon Weston-Broome Robert Faucheaux Jerry Luke LeBlanc Gary Smith Beverly Bruce Dan Flavin Daniel Martiny Jack Smith Peppi Bruneau Mickey Frith Charles McDonald Jane Smith Tom Capella Mike Futrell Tom McVea Vic Stelly Karen Carter Rick Gallot Billy Montgomery Michael Strain Robby Carter Cedric Glover Arthur Morrell Patrick Swilling Don Cazayoux Kyle Green Dan Morrish Francis Thompson Carl Crane Elcie Guillory Edwin Murray Joseph Toomy A.G. Crowe Bryant Hammett Ben Nevers Warren Triche Israel Curtis Herman Hill Ken Odinet Jim Tucker N.J. Damico Avon Honey Tony Perkins Wayne Waddell William Daniel Charles Hudson Rosalind Peychaud Monica Walker Carla Dartez Willie Hunter Wilfred Pierre Michael Walsworth John ‘Juba’ Diez Nita Hutter Loulan Pitre Yvonne Welch Jean Doerge Lydia Jackson Tank Powell Their support of Senate Bill 901 authored by Senator Jon Johnson gives communities the ability to protect residents from the harmful effects of secondhand smoke. -

Aftermath Said
L SEPTEMBER/ OCTOBER Daily news updates 2008 www.ble-t.org ocomotive LEngineers& Trainmen News Published by the BLET, a division of the Rail Conference,• International Brotherhood of Teamsters ELECTION 2008 John McCain’s health care plan proposal a disaster for BLET members, absentee voting information, legislative board endorsements and more. pgs.8 - 11 Deadly Hurricanes Flexible Ike and Gustav ravaged Louisiana and Texas. Spending Account will provide real savings egistration for the money- saving Flexible Spending Account (FSA) begins next month, and BLET mem- bers are encouraged to take advantage of this valuable new health Rand welfare benefit. The program al- lows BLET members to pay for doctor co-pays and other medical expenses with pretax dollars. A partial list of items that qualify for this type of payment includes: Doctor co- pays; Drug Co-pays; Dental Co-pays or other dental expenses not covered in our dental plan; Vision Co-pays or addition- al cost for eye care not covered under the national plan; and Over the counter med- Disaster Relief ications, such as cold medicine, decon- gestant medicine, aspirin, Tylenol or their generic brands. At the Union Pacific Railroad, South- ern Region General Chairman Gil Gore is a staunch supporter of the program. He encourages all BLET members to ke’s sign up. I “I have a personal experience with the medical portion of the FSA,” Brother Gore Aftermath said. “The program has helped tremen- Many BLET members are rebuilding their lives dously with braces and other major den- tal work for my children not covered by with assistance from other BLET members our health insurance.” and the Teamsters Disaster Relief fund. -
Special Election Dates
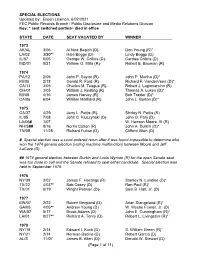
SPECIAL ELECTIONS Updated by: Eileen Leamon, 6/02/2021 FEC Public Records Branch / Public Disclosure and Media Relations Division Key: * seat switched parties/- died in office STATE DATE SEAT VACATED BY WINNER 1973 AK/AL 3/06 Al Nick Begich (D)- Don Young (R)* LA/02 3/20** Hale Boggs (D)- Lindy Boggs (D) IL/07 6/05 George W. Collins (D)- Cardiss Collins (D) MD/01 8/21 William O. Mills (R)- Robert E. Bauman (R) 1974 PA/12 2/05 John P. Saylor (R)- John P. Murtha (D)* MI/05 2/18 Gerald R. Ford (R) Richard F. VanderVeen (D)* CA/13 3/05 Charles M. Teague (R)- Robert J. Lagomarsino (R) OH/01 3/05 William J. Keating (R) Thomas A. Luken (D)* MI/08 4/16 James Harvey (R) Bob Traxler (D)* CA/06 6/04 William Mailliard (R) John L. Burton (D)* 1975 CA/37 4/29 Jerry L. Pettis (R)- Shirley N. Pettis (R) IL/05 7/08 John C. Kluczynski (D)- John G. Fary (D) LA/06# 1/07 W. Henson Moore, III (R) NH/S## 9/16 Norris Cotton (R) John A. Durkin (D)* TN/05 11/25 Richard Fulton (D) Clifford Allen (D) # Special election was a court-ordered rerun after it was found impossible to determine who won the 1974 general election (voting machine malfunction) between Moore and Jeff LaCaze (D). ## 1974 general election between Durkin and Louis Wyman (R) for the open Senate seat was too close to call and the Senate refused to seat either candidate. Special election was held in September 1975. -

Louisiana Bar Journal/April/May 2015
RESURRECTION OF A REAL RIGHT: Annuities with a Charge Also Inside: • Interstate Discovery Simplified • Are Medicare Set-Asides Legally Mandated in Personal Injury Settlements? • Book Review: Acadie LegierCo haystack NO Mag Aug09 8/12/09 4:37 PM Page 1 The Needle In A Haystack Ask how we can help you file BP Oil Spill Claims utilizing the latest court-supervised settlement methodology. Complex financial litigation cases often require the engagement of experts who can find “the needle in a haystack.” A substantial edge is gained when you have Legier & Company’s Forensic & Investigative CPA and Expert Witness Group on your team to help you find obscured financial facts that build and prove stronger cases. Expert Testimony • Fraud • Forensic & Investigative Accounting • Calculating and Refuting Financial Damages Business Valuations • Bankruptcies • Shareholder Disputes • Lost Profits • Business Interruptions Lost Wages • Corporate Veil Piercing • Marital Dissolutions For more information, contact William R. Legier (504) 599-8300 1100 Poydras Street • 34th Floor • Energy Centre • New Orleans, LA 70163 Telephone (504) 561-0020 • Facsimile (504) 561-0023 • http://www.legier.com Louisiana Bar Journal Vol. 62, No. 6 417 418 April / May 2015 ® April / May 2015 Volume 62, Number 6 Departments Features Editor’s Message .................. 423 Interstate Discovery Simplified: Louisiana Passes the Uniform Interstate Depositions and Discovery Act President’s Message ............ 424 By Christopher D. Cazenave and Graham H. Ryan ..............................................426 Association Actions .............. 440 Practice Management........... 450 Resurrection of a Real Right: Annuities with a Charge By Tyler G. Storms .................................................................................................430 Lawyers Assistance .............. 451 Focus on Diversity ................ 452 Are Medicare Set-Asides Legally Mandated in Personal Injury Settlements? Puzzle .................................. -

FALCON V, LLC, Et Al., DEBTORS. CHAPTER 11 CASE NO. 19-105
Case 19-10547 Doc 369 Filed 08/23/19 Entered 08/23/19 15:03:33 Page 1 of 1 UNITED STATES BANKRUPTCY COURT MIDDLE DISTRICT OF LOUISIANA IN RE: CHAPTER 11 FALCON V, L.L.C., et al.,1 CASE NO. 19-10547 DEBTORS. JOINTLY ADMINISTERED CERTIFICATE OF SERVICE Attached hereto is the Affidavit of Service of Jennifer S. Goods of Donlin, Recano & Company, Inc. (the “Affidavit”) which declares that a copy of the Amended Order Approving Disclosure Statement (P-356) was served on the parties listed in Exhibit 1 to the Affidavit on August 21, 2019. Dated: August 23, 2019 Respectfully submitted, KELLY HART PITRE /s/ Louis M. Phillips Patrick (Rick) M. Shelby (#31963) Louis M. Phillips (#10505) Amelia L. Bueche (#36817) One American Place 301 Main Street, Suite 1600 Baton Rouge, LA 70801-1916 Telephone: (225) 381-9643 Facsimile: (225) 336-9763 Email: [email protected] Email: [email protected] Email: [email protected] Counsel for the Debtors 1 The “Debtors” are the following entities (the corresponding bankruptcy case numbers follow in parentheses): Falcon V, L.L.C. (Case No. 19-10547), ORX Resources, L.L.C. (Case No. 19-10548), and Falcon V Holdings, L.L.C. (Case No. 19-10561). The address of the Debtors is 400 Poydras Street, Suite 1100, New Orleans, Louisiana 70130. 1 Case 19-10547 Doc 369-1 Filed 08/23/19 Entered 08/23/19 15:03:33 Page 1 of 286 Case 19-10547 Doc 369-1 Filed 08/23/19 Entered 08/23/19 15:03:33 Page 2 of 286 . -

Barry Ivey Elected to Louisiana House
Baton Rouge’s CAPITALCAPITAL CITYCITY Community Newspaper ForFor NewsNews Updates,Updates, ‘Like’‘Like’ CapitalCapitalon® FACEBOOK CityCity NewsNews NEWSNEWS® Thursday, March 7, 2013 • Vol. 22, No. 4 • 16 Pages • www.capitalcitynews.us • Phone 225-261-5055 Capital Radio Wars — Part II — Country Country Radio War Country Music: Radio Format That’s Uniquely All-American Woody Jenkins Editor, Capital City News BATON ROUGE — While classical music, opera, and rock and roll are worldwide phenomena, country music is uniquely an American invention. Country music is “Made in the USA!” and Photo by Woody Jenkins Woody by Photo country music fans love the Jenkins Woody by Photo Red, White, and Blue. In 1962, a crusty conser- Sam McQuire at WYNK, 101.5 vative businessman named Gordy Rush of The Tiger 100.7 and Country Legends Bob McGregor launched WYNK, the first country Since 1962, WYNK Has music radio station in the Guaranty Offers Market Baton Rouge market. Today, country music is Been BR Country Giant probably the most popular Two BR Country Stations BATON ROUGE — Bob sively stronger each year. format on radio, and that is BATON ROUGE — For still comes to work at the McGregor launched WYNK Sam McQuire, the mas- also true in Baton Rouge. nearly 50 years, Guaranty company’s headquarters on in 1962 and brought along termind behind much of Baton Rouge has three Broadcasting — now Guar- Government Street — the his conservative values and WYNK’s current success, country music stations — anty Media Ventures — has old Baton Rouge General love of country music. Ba- says the goal of the station WYNK, now owned by been a force in Baton Rouge Hospital. -

Local 614 Members Build Submarine in Record Time THANKS to the Efforts of Local 614 Mem- the Submarine Was Christened by Cheryl Mcguin- Bers (Groton, Conn.), the U.S
Vol. 47 No. 3 the Boilermaker Jul • Sep 2008 The Official Publication of the International Brotherhood of Boilermakers, Iron Ship Builders, Blacksmiths, Forgers, and Helpers, AFL-CIO http://capwiz.com/boilermaker Reporter http://www.boilermakers.org IN THESE PAGES Local 614 members build submarine in record time THANKS TO THE efforts of Local 614 mem- The submarine was christened by Cheryl McGuin- bers (Groton, Conn.), the U.S. Navy christened its ness, a resident of Portsmouth, N.H. Her husband, newest Virginia-class nuclear-powered attack Tom, died on Sept. 11, 2001, in the attacks on the submarine, the New Hampshire, on June 21, a date World Trade Center. He was a co-pilot of American that coincided with the 220th anniversary of New Airlines Flight 11. Hampshire’s statehood. The christening marked the third time the U.S. The fifth Virginia class to be built, she was com- Navy has named a ship after the state of New Hamp- pleted months ahead of schedule at General Dynam- shire. The newest New Hampshire is the first Virginia- ics Electric Boat in Groton, Conn. Election 2008 nears . .3 See SUBMARINE, page 2 Pinault tops Canadian event . 10 MOST makes safety videos . .15 The New Hampshire is moored to the pier at the General Dynamics Electric Boat shipyard before her christening June 21. (U.S. Navy photo by John Narewski, courtesy of navy.mil.) MN 100 Org. Org. No. PAID POSTAGE Prairie, REQUESTED PAID POSTAGE NASSCO workers ratify first U.S. Permit Non-Profit U.S. Long Non-Profit SERVICE agreement, settle lawsuit Local 1998-represented employees tional Vice President – Industrial Sector, said it was recoup nearly $14 million for lost clear that NASSCO wished to resolve the lawsuit that had been filed in December 2004. -

Congressional Record United States Th of America PROCEEDINGS and DEBATES of the 110 CONGRESS, SECOND SESSION
E PL UR UM IB N U U S Congressional Record United States th of America PROCEEDINGS AND DEBATES OF THE 110 CONGRESS, SECOND SESSION Vol. 154 WASHINGTON, TUESDAY, MAY 6, 2008 No. 74 House of Representatives The House met at 10:30 a.m. and was running for the Senate, our former col- most people in the House and the Sen- called to order by the Speaker pro tem- league, Rick Lazio, called for sus- ate. pore (Mr. PASTOR). pending the 18.3 cent Federal gas tax And, of course, the irony as Senator and actually repealing the 4.3 cent per f CLINTON herself intimated is that this gallon surcharge that had been en- gas tax holiday is actually a holiday DESIGNATION OF SPEAKER PRO acted. ‘‘What Mrs. CLINTON needs to for the big producers, refiners and im- TEMPORE do,’’ he said, ‘‘is get out of the motor- porters. They’re the ones who pay the The SPEAKER pro tempore laid be- cade, get out of fantasyland and get in tax. The tax is charged to them. In fore the House the following commu- contact with the issues that are affect- nication from the Speaker: ing real New Yorkers, the prices at the order for any of the savings to trickle down to the pockets of motorists, the WASHINGTON, DC, pump.’’ May 6, 2008. It’s instructive what then candidate oil and gas interests would have to de- I hereby appoint the Honorable ED PASTOR CLINTON had to say. She and her aides cide that they’re going to pass their to act as Speaker pro tempore on this day. -

Evidence from Campaign Contributions to Close Congressional Elections ∗
Valuing Changes in Political Networks: Evidence from Campaign Contributions to Close Congressional Elections ∗ Pat Akey † Job Market Paper Current Draft: November 2013 First Draft: February 2012 ABSTRACT This paper investigates the value of firm political connections to US congressional candidates using a regression discontinuity design. In a sample of close special elections occurring at times unrelated to firm-specific economic events or broader political events, I compare the abnormal returns of firms that contributed to winning candidates to those of firms that contributed to losing candidates. I find the wedge between these firms to be 1.7% to 6.8% of firm equity value. To assess which areas of policy matter most, I test which congressional committee assignment seats are the most valuable. In particu- lar, the loss of a connection to the Senate Appropriations committee leads to a loss of $1.9 billion in sales in the following year. Finally, I examine additional actions that firms take to develop political networks—directly hiring former government employees and en- gaging professional lobbyists—and find that these actions complement their contribution strategies. ∗I would like to thank Morten Bennedsen, Atif Ellahie, Sapnoti Eswar, Julian Franks, Vito Gala, Francisco Gomes, Denis Gromb, Jan Jindra, Simon Johnson, Brandon Julio, Steve Karolyi, Anton Lines, Ted Liu, Ste- fan Lewellen, Amine Ouazad, Alexei Ovtchinnikov, Chris Pantzalis, Chris Parsons, Rodney Ramcharan, Oleg Rubanov, Henri Servaes, Jordan Siegel, Elena Simintzi, Janis Sktrastins, Irem˙ Tuna, Vikrant Vig, Paolo Volpin, and Alminas Zaldokas,ˇ along with seminar participants at the 2013 AFA annual meetings, London Business School, INSEAD, the 2013 USC Finance PhD Conference, the 2013 Transatlantic Doctoral Conference, and the 2013 FMA Europe conference, University of Iowa, and the FMA Annual meetings for helpful comments and suggestions. -

History of Legislation
ONE HUNDRED TENTH CONGRESS CONVENED JANUARY 3, 2007 FIRST SESSION ADJOURNED DECEMBER 19, 2007 CONVENED JANUARY 15, 2008 SECOND SESSION ADJOURNED DECEMBER 19, 2008 JOURNAL AND HISTORY OF LEGISLATION UNITED STATES HOUSE OF REPRESENTATIVES COMMITTEE ON FINANCIAL SERVICES BARNEY FRANK, Chairman FINAL EDITION — December 19, 2008 SECOND SESSION For sale by the Superintendent of Documents, Congressional Sales Office U.S. Government Printing Office, Washington, DC 20402 COMMITTEE ON FINANCIAL SERVICES (RATIO: 37–33) BARNEY FRANK, Massachusetts, Chairman PAUL E. KANJORSKI, Pennsylvania SPENCER BACHUS, Alabama MAXINE WATERS, California DEBORAH PRYCE, Ohio CAROLYN B. MALONEY, New York MICHAEL N. CASTLE, Delaware LUIS V. GUTIERREZ, Illinois PETER T. KING, New York NYDIA M. VELÁZQUEZ, New York EDWARD R. ROYCE, California MELVIN L. WATT, North Carolina FRANK D. LUCAS, Oklahoma GARY L. ACKERMAN, New York RON PAUL, Texas BRAD SHERMAN, California STEVEN C. LATOURETTE, Ohio GREGORY W. MEEKS, New York DONALD A. MANZULLO, Illinois DENNIS MOORE, Kansas WALTER B. JONES, JR. North Carolina MICHAEL E. CAPUANO, Massachusetts JUDY BIGGERT, Illinois RUBÉN HINOJOSA, Texas CHRISTOPHER SHAYS, Connecticut WM. LACY CLAY, Missouri GARY G. MILLER, California CAROLYN MCCARTHY, New York SHELLEY MOORE CAPITO, West Virginia JOE BACA, California TOM FEENEY, Florida STEPHEN F. LYNCH, Massachusetts JEB HENSARLING, Texas BRAD MILLER, North Carolina SCOTT GARRETT, New Jersey DAVID SCOTT, Georgia GINNY BROWN-WAITE, Florida AL GREEN, Texas J. GRESHAM BARRETT, South Carolina EMANUEL CLEAVER, Missouri JIM GERLACH, Pennsylvania MELISSA L. BEAN, Illinois STEVAN PEARCE, New Mexico GWEN MOORE, Wisconsin RANDY NEUGEBAUER, Texas LINCOLN DAVIS, Tennessee TOM PRICE, Georgia PAUL W. HODES, New Hampshire GEOFF DAVIS, Kentucky KEITH ELLISON, Minnesota PATRICK MCHENRY, North Carolina RON KLEIN, Florida JOHN CAMPBELL, California TIM MAHONEY, Florida ADAM H. -

State of Louisiana
STATE OF LOUISIANA OFFICE OF STATE INSPECTOR GENERAL Stephen B. Street, Jr., State Inspector General Annual Report For the 12 Months Ending June 30, 2011 Date Issued: January 18, 2012 Table of Contents Transmittal Letter to Governor Jindal and Members of the Joint Legislative Committee on the Budget Introduction 1 History of the Office 2 Resources and Staffing 2 Organization Chart 4 Investigative and Audit Processes 5 State Inspector General Speaking Engagements 6 Analysis of Complaints 8 Case Highlights 9 Page 1 2011 Annual Report Introduction I n keeping with its statutory designation as a law enforcement agency with a mission to root out fraud and corruption in government, the Office of State Inspector General has implemented significant changes over the past year. The office has been reorganized according to the law enforcement model, with criminal investigators and forensic auditors working together as a team on serious fraud and public corruption cases. OIG has now completely shifted its focus away from being an agency that primarily generates reports, and now emphasizes investigations of white-collar criminal activity to be presented for prosecution. As a law enforcement agency, the office is statutorily authorized to access confidential criminal databases maintained by the FBI and Louisiana State Police, can obtain and execute criminal search warrants, obtain criminal arrest warrants and apply for and serve criminal investigative subpoenas. OIG criminal investigators are experienced law enforcement officers trained and certified under Peace Officer Standards and Training (POST) to carry firearms, and have received Special Officer Commissions from the Louisiana State Police. OIG forensic auditors are Certified Inspector General Auditors (CIGA) with extensive experience conducting audits focusing on areas within state government at risk for fraud and corruption.