Colloidal and Deposited Products of the Interaction of Tetrachloroauric Acid with Hydrogen Selenide and Hydrogen Sulfide in Aqueous Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Facile Large Scale Solution Synthesis of Nanostructured Iron, Nickel and Cobalt Telluride and Possible Applications Sungbum Hong Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2017 Facile large scale solution synthesis of nanostructured iron, nickel and cobalt telluride and possible applications Sungbum Hong Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Chemical Engineering Commons, Materials Science and Engineering Commons, Mechanics of Materials Commons, and the Nanoscience and Nanotechnology Commons Recommended Citation Hong, Sungbum, "Facile large scale solution synthesis of nanostructured iron, nickel and cobalt telluride and possible applications" (2017). Graduate Theses and Dissertations. 15321. https://lib.dr.iastate.edu/etd/15321 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Facile large scale solution synthesis of nanostructured iron, nickel and cobalt telluride and possible applications by Sungbum Hong A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Chemical Engineering Program of Study Committee: Yue Wu, Major Professor Zengyi Shao Xinwei Wang The student author and the program of study committee are solely responsible for the content of this thesis. The Graduate College will ensure this thesis is globally accessible and will not permit alterations after a degree is conferred. Iowa State University Ames, Iowa 2017 Copyright © Sungbum Hong, 2017. All rights reserved. ii DEDICATION In memory of my Father Hong, Neung-pyo and Mother Kwon, Kyung-hee iii TABLE OF CONTENTS Page LIST OF FIGURES .................................................................................................. -

Thin Film Deposition Methods for Cuinse2 Solar Cells
Critical Reviews in Solid State and Materials Sciences, 30:1–31, 2005 Copyright c Taylor and Francis Inc. ISSN: 1040-8436 print DOI: 10.1080/10408430590918341 Thin Film Deposition Methods for CuInSe2 Solar Cells Marianna Kemell,∗ Mikko Ritala, and Markku Leskel¨a Laboratory of Inorganic Chemistry, Department of Chemistry, University of Helsinki, Helsinki, Finland CuInSe2 and its alloys with Ga and/or S are among the most promising absorber materials for thin film solar cells. CuInSe2-based solar cells have shown long-term stability and the highest conversion efficiencies of all thin film solar cells, above 19%. Solar cells based on these materials are also very stable, thus allowing long operational lifetimes. The preparation of a thin film solar cell is a multistage process where every step affects the resulting cell performance and the production cost. CuInSe2 and other Cu chalcopyrites can be prepared by a variety of methods, ranging from physical vapor deposition methods such as evaporation and sputtering to low- temperature liquid phase methods such as electrodeposition. The present review discusses first the concept and operation principle of thin film solar cells, as well as the most important thin film solar cell materials. Next, the properties of CuInSe2 and related compounds, as well as features of solar cells made thereof are reviewed. The last part of the text deals with deposition methods used for the preparation of CuInSe2 and Cu(In,Ga)Se2 thin film absorbers and solar cells. Although the emphasis here is on absorber preparation methods, buffer and conducting oxide preparation are discussed as well. Keywords photovoltaics, copper chalcopyrites, absorber layer, buffer layer, transparent conducting oxide Table of Contents LIST OF SYMBOLS AND ABBREVIATIONS ............................................................................................................. -

Mossbauer Spectroscopy of the Ag-Au Chalcogenides Petzite, Fischesserite
Canadian Mineralogist Vol. 30, pp.327-333(1992) MoSSBAUERsPEcrRoscoPY oF THEAs-Au GHALGoGENIDES PETZITE,FISCHESSERITE AND UYTENBOGAARDTITE FRIEDRICH E. WAGNER Physik-DepartmentEIS, TechnischeUniversittit Milnchen, D-8046 Garching, Germany JERZY A. SAWICKI AECL Research,Cholk RiverLaboratories, Chalk River, Ontario KOJ IJO JOSEPHFRIEDL Physik-DepartmentEt|, TechnischeUniversitdt Milnchen, D-8M6 Garching,Germany JOSEPHA. MANDARINO Departmentof Mineralogy'Royal OntarioMuseum, Toronto, OntarioM5S 2C6 DONALD C. HARRIS ologicalSurvey of Canada,601 Booth Street,Ottawa, Ontario KIA OE8 ABSTRACT 1974u M<lssbauerspectra of the77.3 keV l rays o1 weremeasured aL4.2K for natural and synthetic-Ag3AuTe2 (petzite),synrh;ic Ag3AuSe2(fischesserite) and syntheticAg3AuS2 (uytenbogaardtite). All compoundsstudied exhibit iarge giadients in eleitric fi6ld at the gold nuclei, notably the laigest so far found in any gold mineral' The_isomer shiits ind electricquadrupole interactions, and in particular the similarity of theseparameters for Ag3AuS2with those of Au2S, suggestthat the gold in the Ag3AuX2 compoundsshould be consideredas monovalent. Keywords:refractory Au minerals, invisible Au, structurally bound Au, Ag-Au chalcogenides,petzite, fischesserite, uy-tenbogaardtite,l97Au Mcissbauerspectroscopy, isomer shift, electric quadrupole interaction, Hollinger mine, Timmins, Hemlo deposit, Ontario. SOMMAIRE leTAu Nous avons 6tudi6les spectresde Mrissbauerdes rayons ,y (77.3 keV) de I'isotope g6n€r€sd 4,2 K et mesur6s sur des 6chantillonsnaturels er synthetiquesde Ag3AuTe2Oetzite), et des 6chantillonssynthdtiques de AgsAuSe2 (fischesserite)et Ag3AuS2(uytenbogaardiite). Touiies compos6sfont preuve d'un gradient intensedans le champ ilectrique auiour diinuclEus des atomesd'or, et en fait le plus intensequi ait 6t6 d6couvertdans une espdceaurifbre. D'aprbs les d€placementsisombres et les interactions6lectriques quadrupolaires, et en particulier la similarit6 de ces parambtresdans le Ag3AuS2avec ceux de Au2S, I'or dans ces composdsAgAuX2 serait monovalent. -

Revised Version the Crystal Structure of Uytenbogaardtite, Ag3aus2, And
Title The crystal structure of uytenbogaardtite, Ag3AuS2, and its relationships with gold and silver sulfides-selenides Authors Bindi, L; Stanley, Christopher; Seryotkin, YV; Bakakin, VR; Pal'yanova, GA; Kokh, KA Date Submitted 2017-03-30 1 1 1237R – revised version 2 The crystal structure of uytenbogaardtite, Ag3AuS2, and its relationships 3 with gold and silver sulfides-selenides 4 1, 2 3,4 5 5 LUCA BINDI *, CHRISTOPHER J. STANLEY , YURII V. SERYOTKIN , VLADIMIR V. BAKAKIN , 3,4 3,4 6 GALINA A. PAL’YANOVA , KONSTANTIN A. KOKH 7 8 1Dipartimento di Scienze della Terra, Università di Firenze, Via G. La Pira 4, I-50121Firenze, Italy 9 2Natural History Museum, Cromwell Road, London SW7 5BD, United Kingdom 3 10 Sobolev Institute of Geology and Mineralogy of the Siberian Branch of the Russian Academy of Sciences, pr. 11 Akademika Koptyuga, 3, Novosibirsk 630090, Russia 4 12 Novosibirsk State University, Pirogova str., 2, Novosibirsk 630090, Russia 5 13 Institute of Inorganic Chemistry, Siberian Branch of the RAS, prosp. Lavrentieva 3, 630090 Novosibirsk, Russia 14 15 * e-mail address: [email protected] 16 17 Abstract 18 The crystal structure of the mineral uytenbogaardtite, a rare silver-gold sulfide, was solved 19 using intensity data collected on a crystal from the type locality, the Comstock lode, Storey 20 County, Nevada (U.S.A.). The study revealed that the structure is trigonal, space group R 3 c, 3 21 with cell parameters: a = 13.6952(5), c = 17.0912(8) Å, and V = 2776.1(2) Å . The refinement 22 of an anisotropic model led to an R index of 0.0140 for 1099 independent reflections. -

(Silver)-Telluride-(Selenide) Mineral Deposits
Geological and Atmospheric Sciences Publications Geological and Atmospheric Sciences 12-2009 Understanding gold-(silver)-telluride-(selenide) mineral deposits Nigel J. Cook University of Adelaide Cristiana L. Ciobanu University of Adelaide Paul G. Spry Iowa State University, [email protected] Panagiotis Voudouris University of Athens participants of IGCP-486 Follow this and additional works at: https://lib.dr.iastate.edu/ge_at_pubs Part of the Geochemistry Commons, Geology Commons, and the Mineral Physics Commons The complete bibliographic information for this item can be found at https://lib.dr.iastate.edu/ ge_at_pubs/354. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Geological and Atmospheric Sciences at Iowa State University Digital Repository. It has been accepted for inclusion in Geological and Atmospheric Sciences Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Understanding gold-(silver)-telluride-(selenide) mineral deposits Abstract Gold-(silver)-telluride (selenide) ores occur as epithermal orogenic and intrusion related deposits. Although Te and Se are chalcophile elements and share geochemical affinity withu, A formation of selenides and other elements Ag-Au require acidic or reducing environments. The thermodynamic stability conditions for Au and Agtellurides and native tellurium indicate an epithermal environment. Analysis of mineral paragenensis, textures and compositional variation in tellurides/selenides suggest petrogenetic processes involving interaction with fluids leading ot Au scavenging and entrapment in tellurides, changes in chemistry/rates of fluid infiltration and attaining equilibrium in a given assemblage. -

Journal of Solid State Chemistry 273 (2019) 243–286
Journal of Solid State Chemistry 273 (2019) 243–286 Contents lists available at ScienceDirect Journal of Solid State Chemistry journal homepage: www.elsevier.com/locate/jssc The development of strategies for nanoparticle synthesis: Considerations MARK for deepening understanding of inherently complex systems☆ ⁎ Jennifer M. Lee1, Rebecca C. Miller1, Lily J. Moloney, Amy L. Prieto Department of Chemistry, Colorado State University, Fort Collins, CO 80523, United States ABSTRACT The last fifty years of solid-state chemistry have produced a wealth of compounds with complex structures, exciting properties, and from those discoveries, an explosion of new technologies. We continue to strive to understand structure-property relationships as well as develop expedient methods to discover and control new materials. Nanoparticle synthesis is one area of synthetic solid-state chemistry that is currently experiencing a significant growth of deepening understanding and increasing sophistication. Herein, we review examples from the literature where insight about a synthesis can be built from to catalyze new discoveries. The focus is on synthetic reports of nanoparticles of increasing complexity. Contents 1. Introduction. ..................................................... 244 2. Connecting parameters of nanoparticle syntheses to reaction mechanism and pathway. 246 2.1. Investigating the formation of anionic, chalcogenide species . 246 2.1.1. Tertiary phosphine chalcogenides: mechanism of chalcogen delivery and reactivity trends. 246 2.1.2. Diorganyl dichalcogenides: reactivity trends and extrapolation from binary to ternary systems . 247 2.1.3. Alkylamine-chalcogenides: chalcogen dissolution and reduction . 248 2.1.4. Multiple roles of 1-dodecanethiol (DDT): sulfur precursor, stabilizing ligand, and solvent . 249 2.1.5. Thiourea and selenourea: chemical decomposition . 249 2.1.6. -

Some Reactions of Phosphorusfluorine Compounds with Platinum Metal Hydride Complexes Philip G. Page Ii Thesis Presented For
ii Some Reactions of Phosphorusfluorine Compounds with Platinum Metal Hydride Complexes Philip G. Page Thesis presented for the degree of Doctor of Philosophy University of Edinburgh Seotember 1983 To my parents Declaration This thesis has not been submitted, in whole, or in part, previously for any degree at any university. All the work is original, under the supervision of Professor E.A.V. Ebsworth; where this is not the case, credit has been duly given. () Table of Contents Acknowledgements Abstract Chapter 1 Introduction References Chapter 2 Experimental Techniques and Instruments N.m.r. Experiments Chemical Reagents Volatile Reagents Metal Complexes Used C. Solvents Used References Chapter 3 Some Reactions of H 2Pt(PCy 3 ) 2 and HPtC1(PCy3) 2 Introduction Some reactions of H 2Pt(?Cy 3 ) 2 2.1 with EX (X = Cl, Br, I) 2.1.1 Earlier work 2.1.2 New work 2.1.3 The spectra and discussion 2.2 with H 2 E (E = 5, Se) 2.2.1 Earlier work 2.2.2 New work and spectra - 2.2.3 Discussion 2.3 with HPF 2E CE = 0, 5, Se) 2.3.1 Preliminary and earlier work 2.3.2 New work 2.3.3 Discussion. (i ) 2.4 with SiH 4 2.4.1 Earlier work 2.4.2 New work 2.4.3 Discussion 2.5 with PF 2 X (X = Cl, Br, I) 2.5.1 Earlier work 2.5.2 New work 2.5.3 Discussion 2.6 with HPF 2 Some of the reactions of HPt(PCy 3 ) 2 X (X = SiH 3 , S1H, SeH, PF 2O, PF 2 S, PF 2 Se) 3.1 Preliminary 3.2 The reactions of HPt(PCy 3 ) 2SiH 3 3.2.1 with HPF 2E (E = 0, S, Se) 3.2.2 with HC1, HBr, HI 3.2.3 with H 2 S and H 2 Se 3.3 The reactionsof HPt(PCy 3 ). -

Catalyst-Free Construction of Versatile
Article Cite This: Macromolecules 2019, 52, 8596−8603 pubs.acs.org/Macromolecules ‑ Catalyst-Free Construction of Versatile and Functional CS2 Based Polythioureas: Characteristics from Self-Healing to Heavy Metal Absorption † † ‡ † Shuang Wu, Ming Luo,*, Donald J. Darensbourg,*, and Xiaobing Zuo † School of Chemistry and Materials Engineering, Changshu Institute of Technology, Changshu, Jiangsu 215500, China ‡ Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States *S Supporting Information ABSTRACT: As typical of sulfur-containing polymers, polythiourea is a promising polymeric material because of its outstanding properties such as self-healing, high refractive index, high dielectric constant, and good coordinating ability to heavy metal ions. However, examples of versatile polythioureas are relatively scarce as a result of the limited methods for their synthesis. Herein, we report a mild and easily accessible strategy for the preparation of polythioureas by a catalyst-free copolymerization of CS2 and diamines in the absence of an inert/anhydrous atmosphere. The copolymerization of 1,8-diamino-3,6-dioxaoctane (DA1) and carbon disulfide was selected as the model reaction for optimizing conditions for the ff fi polymerization process. DA1 and CS2 a orded well-de ned polythiourea P1 with high molecular weight (25.5 kg/mol) in good yield (96%) at 45 °C, which was shown to be mechanically robust and readily self-healable. This method displayed a wide scope, providing 23 polythioureas with structural diversity and high molecular weights in excellent yields from CS2 and commercially available diamines. The aliphatic polythiourea P4 was examined for its ability as a heavy metal absorbent, effectively sequestering Hg2+ ions with greater than 99.9% efficiency. -

Hydrothermal Studies on Mineral Replacement Reaction in the Gold-Silver-Tellurium and Copper-Iron-Sulphur Systems
HYDROTHERMAL STUDIES ON MINERAL REPLACEMENT REACTIONS IN THE GOLD-SILVER- TELLURIUM AND COPPER-IRON-SULFR SYSTEMS JING ZHAO !" !#$% &'"( "%" !) " *+$# , ) " )#" TABLE OF CONTENTS ABSTRACT .............................................................................................................................. v DECLARATION .................................................................................................................. vii ACKNOWLEDGEMENTS .................................................................................................. ix CHAPTER 1INTRODUCTION .......................................................................................... 1 1.1 Mineral replacement reaction and coupled dissolution reprecipitation mechanism ......... 4 1.1.1 Pseudomorphism .................................................................................................................. 8 1.1.2 Porosity and fine cracks .................................................................................................... 10 1.2 Mineral replacement reactions among gold-(silver)-tellurides ....................................... 13 1.3 Mineral replacement reactions among copper iron sulfides ........................................... 17 1.4 Research objects .............................................................................................................. 23 1.5 References ....................................................................................................................... 24 CHAPTER -

Synthesis of Manganese(II) Containing Metal Chalcogen Cluster Complexes from Metal Trimethylsilylthiolate Precursors
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 2-7-2017 12:00 AM Synthesis of Manganese(II) Containing Metal Chalcogen Cluster Complexes from Metal Trimethylsilylthiolate Precursors Kyle NW Rozic The University of Western Ontario Supervisor John F. Corrigan The University of Western Ontario Graduate Program in Chemistry A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Kyle NW Rozic 2017 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Inorganic Chemistry Commons Recommended Citation Rozic, Kyle NW, "Synthesis of Manganese(II) Containing Metal Chalcogen Cluster Complexes from Metal Trimethylsilylthiolate Precursors" (2017). Electronic Thesis and Dissertation Repository. 4468. https://ir.lib.uwo.ca/etd/4468 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract The manganese(II)-palladium(II)-sulfide complex [MnCl2(µ3-S)2Pd2(dppp)2] 2 has been isolated from the reaction of [(dppp)PdCl2] with [Li(N,N’-tmeda)]2[Mn(SSiMe3)4] 1 in a 2:1 ratio under mild conditions. The trimethylsilyl thiolate complex [(dppp)Pd(SSiMe3)2] 3 has been synthesized from the reaction of [(dppp)PdCl2] with Li[SSiMe3] as well as the reaction of [(dppp)Pd(OAc)2] with Li[SSiMe3] under mild conditions. The newly synthesized complex [(dppp)Pd(SSiMe3)2] 3 was used in reaction with the manganese(II) salt [(CH3CN)2Mn(OTf)2] to form the manganese(II)-palladium(II)-sulfide complex [Mn(OTf)(thf)2(µ-S)2Pd2(dppp)2]OTf 4. -

Silver and Gold Coating
Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org Gold & Silver Coatings By A . T . Kakhia 1 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org 2 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org Part One General Knowledge 3 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org 4 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org Aqua Regia ( Royal Acid ) Freshly prepared aqua regia is colorless, Freshly prepared aqua but it turns orange within seconds. Here, regia to remove metal fresh aqua regia has been added to these salt deposits. NMR tubes to remove all traces of organic material. Contents 1 Introduction 2 Applications 3 Chemistry 3.1 Dissolving gold 3.2 Dissolving platinum 3.3 Reaction with tin 3.4 Decomposition of aqua regia 4 History 1 - Introduction Aqua regia ( Latin and Ancient Italian , lit. "royal water"), aqua regis ( Latin, lit. "king's water") , or nitro – hydro chloric acid is a highly corrosive mixture of acids, a fuming yellow or red solution. The mixture is formed by freshly mixing concentrated nitric acid and hydro chloric acid , optimally in a volume ratio of 1:3. It was named 5 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org so because it can dissolve the so - called royal or noble metals, gold and platinum. However, titanium, iridium, ruthenium, tantalum, osmium, rhodium and a few other metals are capable of with standing its corrosive properties. IUPAC name Nitric acid hydro chloride Other names aqua regia , Nitro hydrochloric acid Molecular formula HNO3 + 3 H Cl Red , yellow or gold Appearance fuming liquid 3 Density 1.01–1.21 g / cm Melting point − 42 °C Boiling point 108 °C Solubility in water miscible in water Vapor pressure 21 mbar 2 – Applications Aqua regia is primarily used to produce chloro auric acid, the electrolyte in the Wohl will process. -
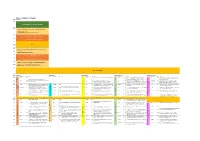
SGA Draft Schedule
Day 1 - Tuesday 27th August Room Bute Hall Time 09:00 Opening Ceremony, Welcome Addresses 09:30 Plenary 1: Geoscience vision for future exploration and resource extraction. Cam McCuaig, Principal Geoscientist, BHP 10:00 Presentation of SGA-NEWMONT Gold Medal 10:15 Presentation of SGA Young Scientist Award 10:30 10:45 Break 11:00 Plenary 2: Responsibility in Mining: How to make difficult 11:15 decisions. Sarah Gordon, Satarla Ltd, UK 11:30 Presentation of SGA-KGHM Krol Medal 11:45 Presentation Mineralium Deposita Best Paper Award 12:00 Plenary 3: Hydrothermal Origins of Brucite, Banded Iron 12:15 Formations, Transition Metal Sulfides and Life Mike Russell, Jet Propulsion Laboratory, CalTec. 12:30 Lunch and Posters 1 Room Bute Hall East Quad LT Kelvin Gallery James Watt S. J15 Stevenson LT (JWS) Time Theme S Presenter* P# Title Theme S Presenter P# Title Theme S Presenter P# Title Theme S Presenter P# Title Theme S Presenter P# Title 14:00 Kay 344 Bauer 180 The structural setting of the Barsele Au deposit, Sweden S Yajioui 13 New occurrence of Ag-Hg-Cu mineralization in the Bath 509 The critical role of architecture in constraining fluid flow Tassafte area, NE edge of the Saghro inlier, Ediacaran- and sustain chemical gradients in Late Archean Gold KEYNOTE: Andean Arc and Backarc Magmatic and Cambrian transition (Eastern Anti-Atlas, Morocco) Systems, eastern Yilgarn Tectonic Processes and the Formation of Giant Cu, Au and 14:15 Kay 344 S Perret 11 The structural control on the gold mineralization at the Li 32 The Xiasai vein-type Ag–Pb–Zn