Business Report Vol.44
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Cleveland, Tn 37311
SPORTS: LOCAL NEWS: Fifth-ranked Raiders Region taking head into district as notice of area top seed: Page 9 growth: Page 13 162nd yEAR • No. 306 16 PAGES • 50¢ CLEVELAND, TN 37311 THE CITY WITH SPIRIT TUESDAY, APRIL 25, 2017 Teachers’ pay concerns voiced Tennessee Crye urges fellow legislators commissioners to give nod to beware of future gas tax hike By BRIAN GRAVES [email protected] One of the Bradley County commissioners took Local projects advantage of an empty agenda Monday night to express his concerns about the pay for county teachers. total $220M Commissioner Thomas Crye said Director of Schools Dr. Linda Cash had advised him she has By BRIAN GRAVES [email protected] been told the county schools will take a $700,000 hit in state BEP funds. The Tennessee House of “All of us should think Representatives voted Monday “I’m not making a our teachers deserve a night to concur with the state howell demand. I’m not minimum pay raise at Senate and give the final making a request. I’m least in the amount of approval to Gov. Bill Haslam’s making an observa- what the Bradley County transportation infrastructure tion and laying it out employees receive,” Crye act. on the table. We have said. The final vote was 67-21. a storm over the hori- County employees The bill prioritizes 962 proj- zon that we need to have gotten 2 percent ects across all of Tennessee’s be aware of.” raises for the last few Banner photo, BRIAN GRAVES 95 counties, addressing a — Commissioner years. -

PLAYERS GUIDE — Shinnecock Hills Golf Club | Southampton, N.Y
. OP U.S EN SHINNECOCK HILLS TH 118TH U.S. OPEN PLAYERS GUIDE — Shinnecock Hills Golf Club | Southampton, N.Y. — June 14-17, 2018 conducted by the 2018 U.S. OPEN PLAYERS' GUIDE — 1 Exemption List SHOTA AKIYOSHI Here are the golfers who are currently exempt from qualifying for the 118th U.S. Open Championship, with their exemption categories Shota Akiyoshi is 183 in this week’s Official World Golf Ranking listed. Birth Date: July 22, 1990 Player Exemption Category Player Exemption Category Birthplace: Kumamoto, Japan Kiradech Aphibarnrat 13 Marc Leishman 12, 13 Age: 27 Ht.: 5’7 Wt.: 190 Daniel Berger 12, 13 Alexander Levy 13 Home: Kumamoto, Japan Rafael Cabrera Bello 13 Hao Tong Li 13 Patrick Cantlay 12, 13 Luke List 13 Turned Professional: 2009 Paul Casey 12, 13 Hideki Matsuyama 11, 12, 13 Japan Tour Victories: 1 -2018 Gateway to The Open Mizuno Kevin Chappell 12, 13 Graeme McDowell 1 Open. Jason Day 7, 8, 12, 13 Rory McIlroy 1, 6, 7, 13 Bryson DeChambeau 13 Phil Mickelson 6, 13 Player Notes: ELIGIBILITY: He shot 134 at Japan Memorial Golf Jason Dufner 7, 12, 13 Francesco Molinari 9, 13 Harry Ellis (a) 3 Trey Mullinax 11 Club in Hyogo Prefecture, Japan, to earn one of three spots. Ernie Els 15 Alex Noren 13 Shota Akiyoshi started playing golf at the age of 10 years old. Tony Finau 12, 13 Louis Oosthuizen 13 Turned professional in January, 2009. Ross Fisher 13 Matt Parziale (a) 2 Matthew Fitzpatrick 13 Pat Perez 12, 13 Just secured his first Japan Golf Tour win with a one-shot victory Tommy Fleetwood 11, 13 Kenny Perry 10 at the 2018 Gateway to The Open Mizuno Open. -

Tournament Field
07/17/17 10:37:27 The Open Championship Ends 07/23/2017 This list is for information purposes only. Field is subject to change. Players can contact PGA TOUR Headquarters for their alternate status. Byeong Hun An Anirban Lahiri Aaron Baddeley Martin Laird Daniel Berger Pablo Larrazabal 156 Players in the Field Alexander Bjork Paul Lawrie Alternates: Adam Bland Tom Lehman Richard Bland Marc Leishman Paul Broadhurst Alexander Levy Wesley Bryan Hao Tong Li Kent Bulle David Lipsky Rafa Cabrera Bello Michael Lorenzo-Vera Laurie Canter Jamie Lovemark Paul Casey Shane Lowry Roberto Castro Joost Luiten Yi Keun Chang Sandy Lyle Kevin Chappell Stuart Manley Luca Cianchetti Prayad Marksaeng Stewart Cink Hideki Matsuyama Darren Clarke Nick McCarthy Austin Connelly Ryan McCarthy John Daly Haydn McCullen Jason Day William McGirt Joseph Dean Rory McIlroy Bryson DeChambeau Maverick McNealy Robert Dinwiddie Phil Mickelson Andrew Dodt Yusaku Miyazato David Drysdale Francesco Molinari Jason Dufner Ryan Moore David Duval Sebastian Munoz Harry Ellis Kevin Na Ernie Els Alex Noren Darren Fichardt Shaun Norris Tony Finau Sean O'Hair Ross Fisher Mark O'Meara Matthew Fitzpatrick Thorbjorn Olesen Tommy Fleetwood Louis Oosthuizen Mark B. Foster Matthieu Pavon Rickie Fowler Pat Perez Ryan Fox Thomas Pieters Dylan Frittelli Alfie Plant Sergio Garcia Ian Poulter Branden Grace Jon Rahm Matthew Griffin Richie Ramsay Emiliano Grillo Patrick Reed Bill Haas Justin Rose Adam Hadwin Xander Schauffele Ashley Hall Charl Schwartzel Todd Hamilton Adam Scott Brian Harman Callum Shinkwin Padraig Harrington Webb Simpson Tyrrell Hatton Cameron Smith Scott Hend Brandt Snedeker Mike Hendry Young-han Song Russell Henley Matthew Southgate Adam Hodkinson Jordan Spieth Charley Hoffman Kyle Stanley J.B. -

Page 01 March 29 A.Indd
ISO 9001:2008 CERTIFIED NEWSPAPER 29 March 2014 28 Jumadal I 1435 - Volume 19 Number 5618 Price: QR2 ON SATURDAY Budget for new fiscal expected tomorrow DOHA: The government is expected to announce its annual budget for the year 2014- 15 tomorrow. The budget for the new fiscal is expected to be one of the largest in the his- tory of Qatar, Al Sharq reported yesterday. The Cabinet approved the draft budget on March 19 before referring it to the Advisory Council for further discussion. At the Cabinet meeting, Minister for Finance H E Ali Sherif Al Emadi explained the aims and objectives of the proposed allocations to various sectors in the new budget. Considering the government’s projected investment in the construction sector, the pri- vate sector is keenly waiting for the budget announcement. Major allocations are expected in the infrastructure, health and education sec- tors this time. Government spending in the current fiscal (2013-14) was an estimated 18 percent higher than in the previous one (2012-13). An estimated 40 percent was allocated to public projects, and salaries and wages increased by 23.7 percent in the current fiscal. Spending on the education sector increased by 15 percent in the current fiscal compared to 2012-13. According to the government’s strategic plans, the new budget is expected to earmark an estimated 13.4 percent of the total allocation, meaning 3.8 percent of the country’s GDP, for BITTER the education sector. Flood-hit vehicle owners ‘can’ seek compensation DOHA: Insurance experts advise that the owners of Wednesday’s flood-hit vehicles can approach the Public Works Authority (Ashghal) or the Ministry of Municipality and Urban Planning for compensation. -

Team HONMA Member Koumei Oda Becomes the Order of Merit Leader
December 2014 Team HONMA member Koumei Oda becomes the Order of Merit leader on the Japan Golf Tour for the first time! Three members of Team HONMA have proven themselves to be winners, thanks in part to the use of Tour World TW727 x Vizard. A first-time feat for HONMA! HONMA GOLF Co., Ltd., (head office: Roppongi Hills Mori Tower 35F, 6-10-1 Roppongi, Minato-ku, Tokyo; representative director & president: Koji Nishitani) signed a contract for the use of HONMA GOLF clubs and accessories with professional golfer Koumei Oda. At the Golf Nippon Series JT Cup, a major Japanese event that was held between December 4th and 7th at the Tokyo Yomiuri Country Club in Tokyo, Oda tied for third place with a 6-under final result. This allowed him to become the top money leader this season among male golfers on the Japan Golf Tour (with total prize earnings of 137,318,693 yen) for the first time in his professional career. For our company as well, this was the first time that a professional golfer under contract with HONMA GOLF secured this honor in our history. Having set out to become the top money leader before the start of the current season, he was fighting hard in pursuit of this goal as one of the leading earners on the tour. Oda used the TW727 series clubs, which are slated to be released in January 2015 (February for markets outside Japan), and notched a series of impressive results to validate the performing attributes of this equipment. He came blazing on the first day with a first-place score of 6-under and no bogeys. -
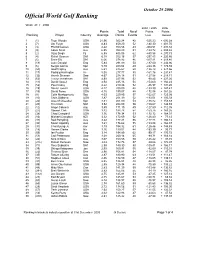
Week 43 Ranking
October 29 2006 Official World Golf Ranking Week 43 / 2006 2004 / 2005 2006 Points Total No of Points Points Ranking Player Country Average Points Events Lost Gained 1 (1) Tiger Woods USA 21.96 922.24 42 -525.23 + 692.28 2 (7) Jim Furyk USA 8.83 459.20 52 -180.30 + 401.70 3 (3) Phil Mickelson USA 8.22 353.56 43 -304.51 + 291.82 4 (9) Adam Scott Aus 6.95 354.29 51 -184.72 + 304.82 5 (2) Vijay Singh Fij 6.55 406.08 62 -480.58 + 270.73 6 (4) Retief Goosen SAf 6.18 352.16 57 -297.85 + 220.66 7 (5) Ernie Els SAf 6.06 278.92 46 -317.91 + 219.40 8 (13) Luke Donald Eng 5.84 291.89 50 -187.00 + 244.96 9 (6) Sergio Garcia Spn 5.79 283.64 49 -228.36 + 179.59 10 (50) Geoff Ogilvy Aus 5.51 275.67 50 -126.72 + 274.66 11 (17) Padraig Harrington Ire 5.05 277.77 55 -161.76 + 231.47 12 (32) Henrik Stenson Swe 4.67 238.18 51 -127.58 + 218.71 13 (62) Trevor Immelman SAf 4.58 237.98 52 -98.43 + 227.26 14 (11) David Howell Eng 4.54 245.16 54 -154.60 + 166.23 15 (52) Paul Casey Eng 4.22 219.46 52 -86.97 + 191.20 16 (19) Davis Love-III USA 4.17 200.00 48 -133.99 + 147.47 17 (15) David Toms USA 4.15 199.07 48 -172.98 + 161.26 18 (8) Colin Montgomerie Sco 4.03 229.85 57 -162.67 + 125.08 19 (10) Chris DiMarco USA 3.87 201.39 52 -183.01 + 150.58 20 (26) Jose M Olazabal Spn 3.81 201.80 53 -136.82 + 159.69 21 (20) Tim Clark SAf 3.52 204.09 58 -158.67 + 148.93 22 (12) Angel Cabrera Arg 3.49 171.19 49 -127.80 + 112.35 23 (27) Stewart Cink USA 3.42 184.78 54 -153.75 + 163.25 24 (38) Chad Campbell USA 3.36 178.34 53 -132.32 + 164.31 25 (16) Michael Campbell NZl 3.32 172.69 -

Asian-Golf-November-2018.Pdf
NEW SOFTEST. LONGEST. STRAIGHTEST. AVAILABLE IN 7 COLORS SOFTEST CLAIM: BASED UPON INTERNAL BALL COMPRESSION TESTING PERFORMED AT WILSON INNOVATION CENTER ON 3/14/17. DISTANCE AND SPIN CLAIMS BASED UPON INTERNAL FLIGHT TESTING PERFORMED AT WILSON’S RESEARCH TEST FACILITY IN HUMBOLDT, TN ON 5/20/17. (90 MPH DRIVER TEST) 218 ISSUE NOVEMBER 2018 Greg Norman who is an old Asia-hand is very bullish about the future of golf in Asia and his assessment of the region is based on solid facts combined with his stepped up busi- ness activity on the Continent. Generally speaking, he is bullish about golf, PERIOD! It is most encouraging to hear a golf great and arguably one of the most respected entrepre- neurs in sports speak about golf with such optimism. It comes as a breath of fresh air after 16 years of the industry languishing in a state of laggardness. Read his full interview inside. Q EQUIPMENT FOCUS 12 POWER FOR THE FEET! Adidas giant has announced a change to the adipower range which essentially incorpo- rates a new forging process as part of the design. This feature is intended to provide extra stability for golfers throughout the swing. 40 A POUND FOR POUND CHAMP! Tour Edge has always been a little guy amongst the heavyweights in the club equip- ment sector of the golf industry. However, it 12 now proudly claims that its new EXS driver has no match – the marketing tagline says it all – “Pound for Pound, Nothing Else Comes Close!” Quite a bombastic boast from a small company! 46 WILSON UPS THE ANTE WITH A NEW BALL! The new Wilson Staff DUO Professional golf ball has been designed to take tour- level performance with great feel to new heights and it is expected to further advance the ongoing success of the famous DUO line. -

Jordan Spieth Grinds out 'Must Win' Over Ikeda
SPORTS SATURDAY, MARCH 25, 2017 MOTOR RACING Hamilton fastest in first practice sessions for Australian GP MELBOURNE: Lewis Hamilton delivered the faster Red Bulls of Daniel Ricciardo and Max Verstappen where we want it to be, but I’m sure we can take a speeds that Formula One rule changes had promised back from third and fourth in the morning session to step forward on that.” yesterday, going under the racing lap record in the fifth and sixth in the afternoon. Ricciardo and Verstappen, who only ran eight laps second practice session for the season-opening Vettel was less than one-tenth of a second in front in the afternoon, were followed by Carlos Sainz of Australian Grand Prix. of Bottas, who replaced Nico Rosberg at Mercedes Toro Rosso, Romain Grossjean - who drove his Haas The three-time world champion had the fastest after the reigning world champion retired. through the gravel during the second session - and time in morning practice and his afternoon time of 1 Hamilton, who won back-to-back world champi- Renault’s Nico Hulkenberg. minute, 23.620 seconds was quicker than the racing onships in 2014 and ‘15 and narrowly lost to Rosberg The new regulations for 2017 allowed for wider lap mark Michael Schumacher set in winning the last season, completed 22 laps in the morning ses- tires with more grip and durability, greater aerody- 2004 title at the 5.303-kilometer (3.295-mile) Albert sion and 34 in the afternoon and used three tire com- namics, bigger fuel loads and increased downforce Park Circuit. -

September 16, 2007
September 16 2007 Official World Golf Ranking Week 37 / 2007 2005 / 2006 2007 Points Total No of Points Points Ranking Player Country Average Points Events Lost Gained 1 (1) Tiger Woods USA 24.36 998.59 41 -542.19 + 689.60 2 (3) Phil Mickelson USA 9.63 423.94 44 -217.74 + 356.54 3 (2) Jim Furyk USA 8.06 443.05 55 -275.34 + 235.36 4 (5) Ernie Els SAf 7.26 384.70 53 -174.62 + 271.61 5 (63) Steve Stricker USA 7.23 303.67 42 -52.32 + 272.04 6 (4) Adam Scott Aus 6.50 331.50 51 -211.81 + 177.56 7 (8) Padraig Harrington Ire 6.11 354.16 58 -166.30 + 211.18 8 (11) Sergio Garcia Spn 5.71 302.74 53 -173.72 + 227.45 9 (39) Rory Sabbatini SAf 5.67 300.73 53 -98.75 + 269.56 10 (29) K.J. Choi Kor 5.63 343.15 61 -114.87 + 277.27 11 (10) Geoff Ogilvy Aus 5.53 276.54 50 -171.25 + 178.60 12 (7) Vijay Singh Fij 5.48 351.02 64 -254.31 + 276.57 13 (51) Justin Rose Eng 5.02 261.23 52 -84.22 + 202.14 14 (12) Henrik Stenson Swe 4.94 252.01 51 -171.66 + 183.22 15 (54) Zach Johnson USA 4.88 263.35 54 -99.65 + 233.63 16 (9) Luke Donald Eng 4.80 244.87 51 -170.24 + 145.64 17 (6) Retief Goosen SAf 4.11 250.55 61 -237.42 + 149.55 18 (13) Trevor Immelman SAf 4.06 215.24 53 -142.17 + 108.40 19 (87) Aaron Baddeley Aus 4.01 220.51 55 -68.68 + 201.78 20 (28) Angel Cabrera Arg 3.99 195.39 49 -101.20 + 141.48 21 (42) Niclas Fasth Swe 3.96 194.07 49 -83.14 + 153.79 22 (15) Paul Casey Eng 3.93 208.03 53 -128.93 + 127.18 23 (26) Stewart Cink USA 3.82 206.20 54 -96.97 + 139.81 24 (52) Scott Verplank USA 3.69 184.25 50 -87.25 + 157.52 25 (44) Arron Oberholser USA 3.58 153.92 43 -

2021 MEDIA GUIDE MARCH 24-28, 2021 AUSTIN COUNTRY CLUB Table of Contents
2021 MEDIA GUIDE MARCH 24-28, 2021 AUSTIN COUNTRY CLUB Table of Contents Format Overview and Glossary .........................................................2 2001 American Express Championship ...................................... 151 Schedule of Events ............................................................................3 2000 American Express Championship ...................................... 152 Media Facts ................................................................................... 4-6 1999 American Express Championship ...................................... 153 Tournament Officials .........................................................................7 FedEx St. Jude Invitational Facts ................................................ 154 2021 Official Scorecard .....................................................................8 2020 FedEx St. Jude Invitational ................................................. 155 4-Year Ranking of Holes at Austin Country Club .............................8 2019 FedEx St. Jude Invitational ................................................. 156 Scoring Averages at a Glance (2016-19) ..........................................9 2018 Bridgestone Invitational ...................................................... 157 Purse Breakdown...............................................................................9 2017 Bridgestone Invitational ...................................................... 158 History at a Glance ........................................................................ -

2018 Staysure Tour Order of Merit 2018
September 3, 2018 Top Ten in the 2018 Race to Dubai Matt Fitzpatrick 1 Francesco MOLINARI (ITA) Pts 4,635,909 2 Patrick REED (USA) 3,057,948 3 Tommy FLEETWOOD (ENG) 2,805,043 4 Rory MCILROY (NIR) 2,760,667 5 Alex NOREN (SWE) 2,729,725 6 Thorbjørn OLESEN (DEN) 2,459,412 7 Jon RAHM (ESP) 2,161,574 8 Russell KNOX (SCO) 2,054,929 9 Tyrrell HATTON (ENG) 1,892,819 10 Rafa CABRERA BELLO (ESP) 1,775,523 This Week on the European Tour Sept 6 - 9, 2018 Omega European Masters Crans-sur-Sierre Golf Club, Crans Montana, Switzerland 2017 CHAMPION: Matt Fitzpatrick (ENG) PRIZE FUND: €2,500,000 DID YOU KNOW? OMEGA EUROPEAN MASTERS • If Matthew Fitzpatrick were to successfully defend his Omega • Crans-sur-Sierre will be staging the tournament for the 72nd European Masters title this week, he would be the first person to do so successive time. Only Augusta National (82 times for the Masters since Seve Ballesteros (1977-78) Tournament), has held the same event more times than the Swiss venue • With his victory last year, Fitzpatrick became the youngest player to reach four European Tour victories, aged 23 years and nine days, since • In 2010, Miguel Angel Jiménez won his first Omega European Masters Matteo Mannassero won the 2014 BMW PGA Championship aged 20 title at the 22nd attempt. At the time, it was a European Tour record for years and 37 days a player competing in the same event the most number of times before winning it for the first time. -

2018 EUROPEAN TOUR FACTS and FIGURES (After Made in Denmark)
2018 EUROPEAN TOUR FACTS AND FIGURES (after Made in Denmark) Low 9 28 (-7) Suttijet Kooratanapisan AfrAsia Bank Mauritius Open 29 (-7) Thorbjørn Olesen BMW International Open 29 (-6) Erik Van Rooyen Dubai Duty Free Irish Open hosted by the Rory Foundation 29 (-6) Brandon Stone, Eddie Pepperell, Patrick Reed, Connor Syme, Aberdeen Standard Investments Scottish Open Matthieu Pavon, Hideto Tanihara 29 (-6) Dustin Johnson WGC-Bridgestone International Low 18 60 (-10) Brandon Stone Aberdeen Standard Investments Scottish Open 61 (-11) Thorbjørn Olesen BMW International Open 61 (-10) Shubhankar Sharma Joburg Open Low 18 to Par 60 (-10) Brandon Stone Aberdeen Standard Investments Scottish Open 61 (-10) Shubhankar Sharma Joburg Open 61 (-11) Thorbjørn Olesen BMW International Open Low 1st 18 62 (-10) Jamie Donaldson Omega Dubai Desert Classic 62 (-9) Arjun Atwal AfrAsia Bank Mauritius Open 62 (-9) Lee Slattery Italian Open 62 (-8) Ian Poulter WGC-Bridgestone Invitational 62 (-8) Clément Sordet Nordea Masters Low 1st 18 to Par 62 (-10) Jamie Donaldson Omega Dubai Desert Classic 62 (-9) Arjun Atwal AfrAsia Bank Mauritius Open 62 (-9) Lee Slattery Italian Open Low 1st 36 129 (-15) Rory McIlroy Omega Dubai Desert Classic 129 (-14) Tapio Pulkkanen Joburg Open 129 (-11) Ian Poulter, Tommy Fleetwood, Justin Thomas WGC-Bridgestone Invitational 129 (-11) Scott Jamieson, Paul Waring Nordea Masters Low 1st 36 to Par 129 (-15) Rory McIlroy Omega Dubai Desert Classic 129 (-14) Tapio Pulkkanen Joburg Open Low Final 36 126 (-16) Justin Thomas WGC-Mexico Championship