LION (Panthera Leo) BOVINE TUBERCULOSIS DISEASE RISK ASSESSMENT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Verzeichnis Der Europäischen Zoos Arten-, Natur- Und Tierschutzorganisationen
uantum Q Verzeichnis 2021 Verzeichnis der europäischen Zoos Arten-, Natur- und Tierschutzorganisationen Directory of European zoos and conservation orientated organisations ISBN: 978-3-86523-283-0 in Zusammenarbeit mit: Verband der Zoologischen Gärten e.V. Deutsche Tierpark-Gesellschaft e.V. Deutscher Wildgehege-Verband e.V. zooschweiz zoosuisse Schüling Verlag Falkenhorst 2 – 48155 Münster – Germany [email protected] www.tiergarten.com/quantum 1 DAN-INJECT Smith GmbH Special Vet. Instruments · Spezial Vet. Geräte Celler Str. 2 · 29664 Walsrode Telefon: 05161 4813192 Telefax: 05161 74574 E-Mail: [email protected] Website: www.daninject-smith.de Verkauf, Beratung und Service für Ferninjektionsgeräte und Zubehör & I N T E R Z O O Service + Logistik GmbH Tranquilizing Equipment Zootiertransporte (Straße, Luft und See), KistenbauBeratung, entsprechend Verkauf undden Service internationalen für Ferninjektionsgeräte und Zubehör Vorschriften, Unterstützung bei der Beschaffung der erforderlichenZootiertransporte Dokumente, (Straße, Vermittlung Luft und von See), Tieren Kistenbau entsprechend den internationalen Vorschriften, Unterstützung bei der Beschaffung der Celler Str.erforderlichen 2, 29664 Walsrode Dokumente, Vermittlung von Tieren Tel.: 05161 – 4813192 Fax: 05161 74574 E-Mail: [email protected] Str. 2, 29664 Walsrode www.interzoo.deTel.: 05161 – 4813192 Fax: 05161 – 74574 2 e-mail: [email protected] & [email protected] http://www.interzoo.de http://www.daninject-smith.de Vorwort Früheren Auflagen des Quantum Verzeichnis lag eine CD-Rom mit der Druckdatei im PDF-Format bei, welche sich großer Beliebtheit erfreute. Nicht zuletzt aus ökologischen Gründen verzichten wir zukünftig auf eine CD-Rom. Stattdessen kann das Quantum Verzeichnis in digitaler Form über unseren Webshop (www.buchkurier.de) kostenlos heruntergeladen werden. Die Datei darf gerne kopiert und weitergegeben werden. -

EPIDEMIOLOGY of SELECTED INFECTIOUS DISEASES in ZOO-UNGULATES: SINGLE SPECIES VERSUS MIXED SPECIES EXHIBITS Carolina Probst
EPIDEMIOLOGY OF SELECTED INFECTIOUS DISEASES IN ZOO-UNGULATES: SINGLE SPECIES VERSUS MIXED SPECIES EXHIBITS Carolina Probst, DVM,* Heribert Hofer, MSc, PhD, Stephanie Speck, DVM, PhD, and Kai Frölich, DVM, PhD1 Institute for Zoo and Wildlife Research, Alfred-Kowalke Strasse 17, 10315 Berlin, Germany Reprinted with permission. American Association of Zoo Veterinarians, 2005. Joint Annual Conference. Abstract The study analyses the epidemiology of selected infectious diseases of 65 different species within the four families of bovids, cervids, camelids and equids in one czech and nine German zoos. It is based on a survey of all epidemiologic data since 1998. Furthermore 900 blood samples taken between 1998 and 2005 are screened for the presence of antibodies against selected viral and bacterial pathogens. The results are linked to the epidemiologic data. Introduction The concept of mixed species exhibits increasingly becomes important in European zoos. It is an important form of behavioral enrichment, it optimizes the use of space and it is of great educational value for visitors, giving them an impression of ecological connections. But until now it has not been elucidated whether the kind of exhibit may lead to an increase in the prevalence of specific infections. The aims of this study are to evaluate the exposure of zoo-ungulates to a variety of disease pathogens that can be transmitted between different species and to assess the epidemiology of mixed exhibits. We are interested in the following questions: 1. Which selected infectious agents are zoo ungulates exposed to? 2. What is the seroprevalence against these agents? 3. Is there a correlation between seroprevalence and the following factors: - animal exhibition system (single species / mixed species exhibit) - population density and animal movements - interspecific contact rates 4. -

Reciprocal Zoos and Aquariums
Reciprocity Please Note: Due to COVID-19, organizations on this list may have put their reciprocity program on hold as advance reservations are now required for many parks. We strongly recommend that you call the zoo or aquarium you are visiting in advance of your visit. Thank you for your patience and understanding during these unprecedented times. Wilds Members: Members of The Wilds receive DISCOUNTED or FREE admission to the AZA-accredited zoos and aquariums on the list below. Wilds members must present their current membership card along with a photo ID for each adult listed on the membership to receive their discount. Each zoo maintains its own discount policies, and The Wilds strongly recommends calling ahead before visiting a reciprocal zoo. Each zoo reserves the right to limit the amount of discounts, and may not offer discounted tickets for your entire family size. *This list is subject to change at any time. Visiting The Wilds from Other Zoos: The Wilds is proud to offer a 50% discount on the Open-Air Safari tour to members of the AZA-accredited zoos and aquariums on the list below. The reciprocal discount does not include parking. If you do not have a valid membership card, please contact your zoo’s membership office for a replacement. This offer cannot be combined with any other offers or discounts, and is subject to change at any time. Park capacity is limited. Due to COVID-19 advance reservations are now required. You may make a reservation by calling (740) 638-5030. You must present your valid membership card along with your photo ID when you check in for your tour. -

Canid, Hye A, Aardwolf Conservation Assessment and Management Plan (Camp) Canid, Hyena, & Aardwolf
CANID, HYE A, AARDWOLF CONSERVATION ASSESSMENT AND MANAGEMENT PLAN (CAMP) CANID, HYENA, & AARDWOLF CONSERVATION ASSESSMENT AND MANAGEMENT PLAN (CAMP) Final Draft Report Edited by Jack Grisham, Alan West, Onnie Byers and Ulysses Seal ~ Canid Specialist Group EARlliPROMSE FOSSIL RIM A fi>MlY Of CCNSERVA11QN FUNDS A Joint Endeavor of AAZPA IUCN/SSC Canid Specialist Group IUCN/SSC Hyaena Specialist Group IUCN/SSC Captive Breeding Specialist Group CBSG SPECIES SURVIVAL COMMISSION The work of the Captive Breeding Specialist Group is made possible by gellerous colltributiolls from the following members of the CBSG Institutional Conservation Council: Conservators ($10,000 and above) Federation of Zoological Gardens of Arizona-Sonora Desert Museum Claws 'n Paws Australasian Species Management Program Great Britain and Ireland BanhamZoo Darmstadt Zoo Chicago Zoological Society Fort Wayne Zoological Society Copenhagen Zoo Dreher Park Zoo Columbus Zoological Gardens Gladys Porter Zoo Cotswold Wildlife Park Fota Wildlife Park Denver Zoological Gardens Indianapolis Zoological Society Dutch Federation of Zoological Gardens Great Plains Zoo Fossil Rim Wildlife Center Japanese Association of Zoological Parks Erie Zoological Park Hancock House Publisher Friends of Zoo Atlanta and Aquariums Fota Wildlife Park Kew Royal Botanic Gardens Greater Los Angeles Zoo Association Jersey Wildlife Preservation Trust Givskud Zoo Miller Park Zoo International Union of Directors of Lincoln Park Zoo Granby Zoological Society Nagoya Aquarium Zoological Gardens The Living Desert Knoxville Zoo National Audubon Society-Research Metropolitan Toronto Zoo Marwell Zoological Park National Geographic Magazine Ranch Sanctuary Minnesota Zoological Garden Milwaukee County Zoo National Zoological Gardens National Aviary in Pittsburgh New York Zoological Society NOAHS Center of South Africa Parco Faunistico "La To:rbiera" Omaha's Henry Doorly Zoo North of Chester Zoological Society Odense Zoo Potter Park Zoo Saint Louis Zoo Oklahoma City Zoo Orana Park Wildlife Trust Racine Zoological Society Sea World, Inc. -

2021 Santa Barbara Zoo Reciprocal List
2021 Santa Barbara Zoo Reciprocal List – Updated July 1, 2021 The following AZA-accredited institutions have agreed to offer a 50% discount on admission to visiting Santa Barbara Zoo Members who present a current membership card and valid picture ID at the entrance. Please note: Each participating zoo or aquarium may treat membership categories, parking fees, guest privileges, and additional benefits differently. Reciprocation policies subject to change without notice. Please call to confirm before you visit. Iowa Rosamond Gifford Zoo at Burnet Park - Syracuse Alabama Blank Park Zoo - Des Moines Seneca Park Zoo – Rochester Birmingham Zoo - Birmingham National Mississippi River Museum & Aquarium - Staten Island Zoo - Staten Island Alaska Dubuque Trevor Zoo - Millbrook Alaska SeaLife Center - Seaward Kansas Utica Zoo - Utica Arizona The David Traylor Zoo of Emporia - Emporia North Carolina Phoenix Zoo - Phoenix Hutchinson Zoo - Hutchinson Greensboro Science Center - Greensboro Reid Park Zoo - Tucson Lee Richardson Zoo - Garden Museum of Life and Science - Durham Sea Life Arizona Aquarium - Tempe City N.C. Aquarium at Fort Fisher - Kure Beach Arkansas Rolling Hills Zoo - Salina N.C. Aquarium at Pine Knoll Shores - Atlantic Beach Little Rock Zoo - Little Rock Sedgwick County Zoo - Wichita N.C. Aquarium on Roanoke Island - Manteo California Sunset Zoo - Manhattan Topeka North Carolina Zoological Park - Asheboro Aquarium of the Bay - San Francisco Zoological Park - Topeka Western N.C. (WNC) Nature Center – Asheville Cabrillo Marine Aquarium -

Local Destinations for Children to Interact with Animals Kansas City
veggies being grown. They may even get to sample those What? The Nature Center is home to that are ripe!** many live, native reptiles, amphibians and fish. The Ernie Miller Nature Center surrounding area also features a www.erniemiller.com bird feeding area, garden pool and Where? 909 N. Highway 7 Olathe, Kansas butterfly garden. When? Monday through Saturday 9 AM to 5 PM, Sunday 1 PM to 5 Why? Classes are offered most Saturdays PM (March 1 through October 31) Monday through Saturday (although most are aimed at children 5 and older), and the Local Destinations for Children to Interact with Animals 9 AM to 4:30 PM, Sunday 12:30 PM to 4:30 PM (November 1 park features a stroller-friendly walking trail and takes visitors Kansas City Zoo through February 28); Closed daily for lunch from 12-1 PM; through meadows, past a variety of wildflowers , and along www.kansascityzoo.org closed on Sundays June, July and August. the creek where children can easily spot native animals. Where? 6800 Zoo Drive (inside Kansas City, Missouri’s Swope Park) What? The Center provides an opportunity for learning, When? Open year round 9:30 AM to 4PM (Closed Thanksgiving Day, understanding, and admiring nature’s ever-changing ways. Anita B. Gorman Discovery Center Christmas Day and New Year’s Day) The Center contains displays and live animals, along with a http://mdc.mo.gov/regions/kansas-city/discovery-center What? Recognized as one of “America’s Best Zoos, “the Kansas City friendly, knowledgeable staff eager to share their knowledge Where? 4750 Troost Avenue, Kansas City, Missouri Zoo is a 202-acre nature sanctuary providing families with of nature. -
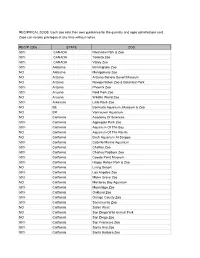
2006 Reciprocal List
RECIPRICAL ZOOS. Each zoo sets their own guidelines for the quantity and ages admitted per card. Zoos can revoke privileges at any time without notice. RECIP 2006 STATE ZOO 50% CANADA Riverview Park & Zoo 50% CANADA Toronto Zoo 50% CANADA Valley Zoo 50% Alabama Birmingham Zoo NO Alabama Montgomery Zoo NO Arizona Arizona-Sonora Desert Museum NO Arizona Navajo Nation Zoo & Botanical Park 50% Arizona Phoenix Zoo 50% Arizona Reid Park Zoo NO Arizona Wildlife World Zoo 50% Arkansas Little Rock Zoo NO BE Bermuda Aquarium, Museum & Zoo NO BR Vancouver Aquarium NO California Academy Of Sciences 50% California Applegate Park Zoo 50% California Aquarium Of The Bay NO California Aquarium Of The Pacific NO California Birch Aquarium At Scripps 50% California Cabrillo Marine Aquarium 50% California Chaffee Zoo 50% California Charles Paddock Zoo 50% California Coyote Point Museum 50% California Happy Hollow Park & Zoo NO California Living Desert 50% California Los Angeles Zoo 50% California Micke Grove Zoo NO California Monterey Bay Aquarium 50% California Moonridge Zoo 50% California Oakland Zoo 50% California Orange County Zoo 50% California Sacramento Zoo NO California Safari West NO California San Diego Wild Animal Park NO California San Diego Zoo 50% California San Francisco Zoo 50% California Santa Ana Zoo 50% California Santa Barbara Zoo NO California Seaworld San Diego 50% California Sequoia Park Zoo NO California Six Flags Marine World NO California Steinhart Aquarium NO CANADA Calgary Zoo 50% Colorado Butterfly Pavilion NO Colorado Cheyenne -

Asiatic Golden Cat in Thailand Population & Habitat Viability Assessment
Asiatic Golden Cat in Thailand Population & Habitat Viability Assessment Chonburi, Thailand 5 - 7 September 2005 FINAL REPORT Photos courtesy of Ron Tilson, Sumatran Tiger Conservation Program (golden cat) and Kathy Traylor-Holzer, CBSG (habitat). A contribution of the IUCN/SSC Conservation Breeding Specialist Group. Traylor-Holzer, K., D. Reed, L. Tumbelaka, N. Andayani, C. Yeong, D. Ngoprasert, and P. Duengkae (eds.). 2005. Asiatic Golden Cat in Thailand Population and Habitat Viability Assessment: Final Report. IUCN/SSC Conservation Breeding Specialist Group, Apple Valley, MN. IUCN encourages meetings, workshops and other fora for the consideration and analysis of issues related to conservation, and believes that reports of these meetings are most useful when broadly disseminated. The opinions and views expressed by the authors may not necessarily reflect the formal policies of IUCN, its Commissions, its Secretariat or its members. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. © Copyright CBSG 2005 Additional copies of Asiatic Golden Cat of Thailand Population and Habitat Viability Assessment can be ordered through the IUCN/SSC Conservation Breeding Specialist Group, 12101 Johnny Cake Ridge Road, Apple Valley, MN 55124, USA (www.cbsg.org). The CBSG Conservation Council These generous contributors make the work of CBSG possible Providers $50,000 and above Paignton Zoo Emporia Zoo Parco Natura Viva - Italy Laurie Bingaman Lackey Chicago Zoological Society Perth Zoo Lee Richardson Zoo -Chairman Sponsor Philadelphia Zoo Montgomery Zoo SeaWorld, Inc. -

Kczoo Reopening to the Public with Precautions
FOR IMMEDIATE RELEASE: May 14, 2020 Contact: Kim Romary, Director of Marketing (816)5 95-1204 desk, (816) 668-4984 cell Josh Hollingsworth, Marketing & Social Media Manager (816) 595-1205 desk, (816) 522-8617 cell [email protected] KCZoo Reopening to the Public with Precautions Kansas City Zoo: Here we come ROARing back! After being closed for more than eight weeks, the Kansas City Zoo will reopen our doors to the public this Saturday, May 16, but with some changes. To ensure the health of our guests, employees, and animals, we are taking precautions that follow national and local guidelines for encouraging physical distancing and reducing high-touch areas. Here is what the public can expect: Limited number of guests • All guests must reserve an entry time at kansascityzoo.org. Entry times will be available in 15- minute increments, up to one week in advance. This includes Friends of the Zoo members and those holding free passes, although there will be no charge associated with making a reservation for these groups. Those without online access can call the Zoo at (816) 595-1234 to reserve a timed ticket. • There will be a limited number of tickets available to keep attendance within the city’s guidelines. Increased social distancing • Daily activities such as shows and Zookeeper Chats are temporarily suspended but are available virtually with a QR code you can access with your mobile phone or through our web site. • Indoor exhibits will be one-way traffic as will pathways around the Africa section of the Zoo and through Australia and Tiger Trail. -

Vereinsinformation Veranstaltungskalender Wilhelma-Treff Tagesfahrt Neues Aus Der Geschäftsstelle 2021 Liebe Mitglieder Des Fördervereins
Vereinsinformation Veranstaltungskalender Wilhelma-Treff Tagesfahrt Neues aus der Geschäftsstelle 2021 Liebe Mitglieder des Fördervereins, das denkwürdige Jahr 2020 hat unseren Blick geschärft für das, was uns alle verbindet. Ganz nach- drücklich zeigen die Entwicklungen der Pandemie – aber auch deren Folgen für die vernetzte Welt- wirtschaft und die internationalen Beziehungen – wie eng verwoben die globalen Zusammenhänge sind. Deshalb dürfen wir uns gerade in einer Zeit, die uns in so vielerlei Hinsicht einschränkt, nicht aufhalten lassen, unsere Vielfalt rund um die Erde zu schützen und die Zukunft gemeinsam zu gestal- ten: mit Unterstützung für Artenschutzprogramme weltweit, aber auch dem Einsatz für den Erhalt der Biodiversität hier bei uns. So durfte der Betrieb der Wilhelma trotz der achtwöchi- gen Schließung keinen Tag ruhen. Die seltenen Tiere und exotischen Pflanzen galt es zu hegen und zu pflegen. Wenn das Leben weitergeht, ergeben sich auch schöne Ereignis- se. Hinter verschlossenen Toren kam im Frühjahr äußerst seltener und daher sehr wertvoller Nachwuchs bei den Okapis, Bonobos und Poitou-Eseln zur Welt. Später folgten zwei Kälber bei den in der Natur bereits ausgestorbenen Säbelantilopen. Liboso mit Sohn Kenai Solange keine Gäste in den Park durften, machten die Bauarbeiter aus der Not eine Tugend und brachten Wegesanierungen schneller voran, als das mit Publikumsverkehr möglich gewesen wäre. Und als im Mai die Wilhelma wieder öffnen durfte, konnten Mädchen und Jungen staunen, dass der neue Hauptspielplatz mit Regenwald-Motto in aller Stille fertig geworden war. Entsprechend der langen Tradition des Zoologischen-Bota- nischen Gartens ist es wieder gelungen, unseren Freundin- nen und Freunden etwas für die Wilhelma komplett Neues zu bieten. -

Population Analysis & Breeding and Transfer Plan Cheetah
Draft for Institutional Representative Review – Please Respond by 3 March 2017 Population Analysis & Breeding and Transfer Plan Cheetah (Acinonyx jubatus) AZA Species Survival Plan® Yellow Program AZA Species Survival Plan® Coordinator Adrienne Crosier, PhD, Smithsonian’s Conservation Biology Institute – Center for Species Survival, ([email protected]) & AZA Studbook Keeper Erin Moloney, Busch Gardens, ([email protected]) AZA Population Advisor John Andrews, Population Management Center ([email protected]) 1 February 2017 Draft for Institutional Representative Review – Please Respond by 3 March 2017 Executive Summary 2017 Species Survival Plan® for the Cheetah (Acinonyx jubatus) The Cheetah SSP population currently consists of 315 cats (151 males, 164 females) at 59 facilities (55 AZA, 4 non- AZA) as of 18 January 2017. Currently, there are thirteen cheetahs (9.4) held at non-AZA facilities. The target population size set by the Felid Taxon Advisory Group is 300 in the 2009 Regional Collection Plan. The population currently qualifies as a Yellow SSP Program. Current gene diversity for the managed population is 97.27% and is equivalent to the genetic diversity of a population descended from approximately 18 founders (FGE = 18.34). The standard genetic goal for AZA managed populations is to maintain 90% gene diversity for 100 years. When gene diversity falls below 90% (and average inbreeding increases), it is expected that reproduction will be increasingly compromised by, among other factors, lower birth weights, smaller litter sizes, and greater neonatal mortality. Under potential conditions, with a growth rate of 0% (lambda = 1.00), gene diversity can be maintained at or above 90% gene diversity for at least 50 years. -

North American Zoos with Mustelid Exhibits
North American Zoos with Mustelid Exhibits List created by © birdsandbats on www.zoochat.com. Last Updated: 19/08/2019 African Clawless Otter (2 holders) Metro Richmond Zoo San Diego Zoo American Badger (34 holders) Alameda Park Zoo Amarillo Zoo America's Teaching Zoo Bear Den Zoo Big Bear Alpine Zoo Boulder Ridge Wild Animal Park British Columbia Wildlife Park California Living Museum DeYoung Family Zoo GarLyn Zoo Great Vancouver Zoo Henry Vilas Zoo High Desert Museum Hutchinson Zoo 1 Los Angeles Zoo & Botanical Gardens Northeastern Wisconsin Zoo & Adventure Park MacKensie Center Maryland Zoo in Baltimore Milwaukee County Zoo Niabi Zoo Northwest Trek Wildlife Park Pocatello Zoo Safari Niagara Saskatoon Forestry Farm and Zoo Shalom Wildlife Zoo Space Farms Zoo & Museum Special Memories Zoo The Living Desert Zoo & Gardens Timbavati Wildlife Park Turtle Bay Exploration Park Wildlife World Zoo & Aquarium Zollman Zoo American Marten (3 holders) Ecomuseum Zoo Salomonier Nature Park (atrata) ZooAmerica (2.1) 2 American Mink (10 holders) Bay Beach Wildlife Sanctuary Bear Den Zoo Georgia Sea Turtle Center Parc Safari San Antonio Zoo Sanders County Wildlife Conservation Center Shalom Wildlife Zoo Wild Wonders Wildlife Park Zoo in Forest Park and Education Center Zoo Montana Asian Small-clawed Otter (38 holders) Audubon Zoo Bright's Zoo Bronx Zoo Brookfield Zoo Cleveland Metroparks Zoo Columbus Zoo and Aquarium Dallas Zoo Denver Zoo Disney's Animal Kingdom Greensboro Science Center Jacksonville Zoo and Gardens 3 Kansas City Zoo Houston Zoo Indianapolis