Proposed SMAC List Effective June 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

Betamethasone Valerate Foam: a Look at the Clinical Data
Review: Clinical Trial Outcomes Betamethasone valerate foam: a look at the clinical data Clin. Invest. (2014) 4(3), 259–267 Topical corticosteroids and especially betamethasone valerate (BMV) have Avner Shemer1, Nicole Sakka1 & been used topically to relieve many inflammatory skin conditions such as Dov Tamarkin*2 psoriasis and atopic dermatitis. The vehicle used to deliver topical drugs 1Department of Dermatology, the Chaim Sheba Medical Center, Affiliated with the can influence the performance of these topical applications. BMV has Tel-Aviv University, Sackler School of Medicine, traditionally been available in creams, ointments, lotions and sprays. In Tel Hashomer, Israel the early 2000s, a topical hydroethanolic BMV foam became commercially 2Foamix Ltd., 2 Holzman Street, Weizmann available. Subsequently, alcohol-free emulsion- and petrolatum-based Science Park, Rehovot 76704, Israel foam formulations were also developed. This manuscript reviews the *Author for correspondence: Tel.: +972 52 457 5677 properties of BMV foams and clinical studies that have been conducted Fax: +972 8 853 1102 to assess their efficacy and safety as treatments for scalp and non-scalp [email protected] psoriasis, as well as other dermatological inflammatory conditions. Keywords: betamethasone valerate • foam • psoriasis • topical corticosteroids Topical corticosteroids have been ranked in four groups consisting of seven classes ranging from ultra-high potency preparations (class 1) to low-potency prepara- tions (class 7). Betamethasone valerate (BMV) is a mid-potency corticosteroid (class 3–5, depending on the dosage form), used topically to relieve inflammatory skin conditions. It is used as a treatment for psoriasis, atopic dermatitis and other corticosteroid-responsive dermatoses. The vehicle used to deliver topical drugs can influence the performance of these drugs. -

4. Antibacterial/Steroid Combination Therapy in Infected Eczema
Acta Derm Venereol 2008; Suppl 216: 28–34 4. Antibacterial/steroid combination therapy in infected eczema Anthony C. CHU Infection with Staphylococcus aureus is common in all present, the use of anti-staphylococcal agents with top- forms of eczema. Production of superantigens by S. aureus ical corticosteroids has been shown to produce greater increases skin inflammation in eczema; antibacterial clinical improvement than topical corticosteroids alone treatment is thus pivotal. Poor patient compliance is a (6, 7). These findings are in keeping with the demon- major cause of treatment failure; combination prepara- stration that S. aureus can be isolated from more than tions that contain an antibacterial and a topical steroid 90% of atopic eczema skin lesions (8); in one study, it and that work quickly can improve compliance and thus was isolated from 100% of lesional skin and 79% of treatment outcome. Fusidic acid has advantages over normal skin in patients with atopic eczema (9). other available topical antibacterial agents – neomycin, We observed similar rates of infection in a prospective gentamicin, clioquinol, chlortetracycline, and the anti- audit at the Hammersmith Hospital, in which all new fungal agent miconazole. The clinical efficacy, antibac- patients referred with atopic eczema were evaluated. In terial activity and cosmetic acceptability of fusidic acid/ a 2-month period, 30 patients were referred (22 children corticosteroid combinations are similar to or better than and 8 adults). The reason given by the primary health those of comparator combinations. Fusidic acid/steroid physician for referral in 29 was failure to respond to combinations work quickly with observable improvement prescribed treatment, and one patient was referred be- within the first week. -

Bluecross Blueshield of Western New York Formulary 1 Please Bring This Guide with You the Next Time You Visit Your Doctor
BlueCross BlueShield of Western New York Formulary 1 Please bring this guide with you the next time you visit your doctor. If you have questions about your prescription drug benefit, visit the Pharmacy Services section of the BlueCross BlueShield web site at www.bcbswny.com. CRP2107_009678.1 MG009678A (Revise Date 07/01/2021) A Division of HealthNow New York Inc. An independent licensee of the BlueCross BlueShield Association. The Cross and Shield are registered trademarks of the BlueCross BlueShield Association. Inside front cover TABLE OF CONTENTS INTRODUCTION . iii UNDERSTANDING THE SYMBOLS USED THROUGHOUT THIS BOOK . iii USING THIS FORMULARY BOOK TO HELP CONTAIN COSTS . iv Save Money on Your Prescription Drugs . iv Finding Medications in the Guide . v SECTION 1 — THERAPEUTIC DRUG CATEGORIES . 1 SECTION II — INDEX . 8 ii The BlueCross BlueShield of Western New York Formulary 1 is a list of drugs to help guide physicians and pharmacists to select the medication that provides the appropriate treatment for the best price. INTRODUCTION BlueCross Blue Shield of Western New York has established an independent committee of practicing physicians and a pharmacist to help ensure that our formularies are medically sound and that they support your patients’ health. This committee—called the Pharmacy and Therapeutics Committee—reviews and evaluates medications on the formulary based on safety and efficacy to help maintain clinical integrity in all therapeutic categories. UNDERSTANDING THE SYMBOLS USED THROUGHOUT THIS BOOK Throughout this book, you will see certain symbols that indicate a management program is in place for selected medications. The symbols are as follows: Key P = A step edit applies to this drug. -

Updates in Pediatric Dermatology
Peds Derm Updates ELIZABETH ( LISA) SWANSON , M D ADVANCED DERMATOLOGY COLORADO ROCKY MOUNTAIN HOSPITAL FOR CHILDREN [email protected] Disclosures Speaker Sanofi Regeneron Amgen Almirall Pfizer Advisory Board Janssen Powerpoints are the peacocks of the business world; all show, no meat. — Dwight Schrute, The Office What’s New In Atopic Dermatitis? Impact of Atopic Dermatitis Eczema causes stress, sleeplessness, discomfort and worry for the entire family Treating one patient with eczema is an example of “trickle down” healthcare Patients with eczema have increased risk of: ADHD Anxiety and Depression Suicidal Ideation Parental depression Osteoporosis and osteopenia (due to steroids, decreased exercise, and chronic inflammation) Impact of Atopic Dermatitis Sleep disturbances are a really big deal Parents of kids with atopic dermatitis lose an average of 1-1.5 hours of sleep a night Even when they sleep, kids with atopic dermatitis don’t get good sleep Don’t enter REM as much or as long Growth hormone is secreted in REM (JAAD Feb 2018) Atopic Dermatitis and Food Allergies Growing evidence that food allergies might actually be caused by atopic dermatitis Impaired barrier allows food proteins to abnormally enter the body and stimulate allergy Avoiding foods can be harmful Proper nutrition is important Avoidance now linked to increased risk for allergy and anaphylaxis Refer severe eczema patients to Allergist before 4-6 mos of age to talk about food introduction Pathogenesis of Atopic Dermatitis Skin barrier -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

St John's Institute of Dermatology
St John’s Institute of Dermatology Topical steroids This leaflet explains more about topical steroids and how they are used to treat a variety of skin conditions. If you have any questions or concerns, please speak to a doctor or nurse caring for you. What are topical corticosteroids and how do they work? Topical corticosteroids are steroids that are applied onto the skin and are used to treat a variety of skin conditions. The type of steroid found in these medicines is similar to those produced naturally in the body and they work by reducing inflammation within the skin, making it less red and itchy. What are the different strengths of topical corticosteroids? Topical steroids come in a number of different strengths. It is therefore very important that you follow the advice of your doctor or specialist nurse and apply the correct strength of steroid to a given area of the body. The strengths of the most commonly prescribed topical steroids in the UK are listed in the table below. Table 1 - strengths of commonly prescribed topical steroids Strength Chemical name Common trade names Mild Hydrocortisone 0.5%, 1.0%, 2.5% Hydrocortisone Dioderm®, Efcortelan®, Mildison® Moderate Betamethasone valerate 0.025% Betnovate-RD® Clobetasone butyrate 0.05% Eumovate®, Clobavate® Fluocinolone acetonide 0.001% Synalar 1 in 4 dilution® Fluocortolone 0.25% Ultralanum Plain® Fludroxycortide 0.0125% Haelan® Tape Strong Betamethasone valerate 0.1% Betnovate® Diflucortolone valerate 0.1% Nerisone® Fluocinolone acetonide 0.025% Synalar® Fluticasone propionate 0.05% Cutivate® Hydrocortisone butyrate 0.1% Locoid® Mometasone furoate 0.1% Elocon® Very strong Clobetasol propionate 0.1% Dermovate®, Clarelux® Diflucortolone valerate 0.3% Nerisone Forte® 1 of 5 In adults, stronger steroids are generally used on the body and mild or moderate steroids are used on the face and skin folds (armpits, breast folds, groin and genitals). -

Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Cr
Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Crizotinib (PF-02341066) 1 4 55 Docetaxel 1 5 98 Anastrozole 1 6 25 Cladribine 1 7 23 Methotrexate 1 8 -187 Letrozole 1 9 65 Entecavir Hydrate 1 10 48 Roxadustat (FG-4592) 1 11 19 Imatinib Mesylate (STI571) 1 12 0 Sunitinib Malate 1 13 34 Vismodegib (GDC-0449) 1 14 64 Paclitaxel 1 15 89 Aprepitant 1 16 94 Decitabine 1 17 -79 Bendamustine HCl 1 18 19 Temozolomide 1 19 -111 Nepafenac 1 20 24 Nintedanib (BIBF 1120) 1 21 -43 Lapatinib (GW-572016) Ditosylate 1 22 88 Temsirolimus (CCI-779, NSC 683864) 1 23 96 Belinostat (PXD101) 1 24 46 Capecitabine 1 25 19 Bicalutamide 1 26 83 Dutasteride 1 27 68 Epirubicin HCl 1 28 -59 Tamoxifen 1 29 30 Rufinamide 1 30 96 Afatinib (BIBW2992) 1 31 -54 Lenalidomide (CC-5013) 1 32 19 Vorinostat (SAHA, MK0683) 1 33 38 Rucaparib (AG-014699,PF-01367338) phosphate1 34 14 Lenvatinib (E7080) 1 35 80 Fulvestrant 1 36 76 Melatonin 1 37 15 Etoposide 1 38 -69 Vincristine sulfate 1 39 61 Posaconazole 1 40 97 Bortezomib (PS-341) 1 41 71 Panobinostat (LBH589) 1 42 41 Entinostat (MS-275) 1 43 26 Cabozantinib (XL184, BMS-907351) 1 44 79 Valproic acid sodium salt (Sodium valproate) 1 45 7 Raltitrexed 1 46 39 Bisoprolol fumarate 1 47 -23 Raloxifene HCl 1 48 97 Agomelatine 1 49 35 Prasugrel 1 50 -24 Bosutinib (SKI-606) 1 51 85 Nilotinib (AMN-107) 1 52 99 Enzastaurin (LY317615) 1 53 -12 Everolimus (RAD001) 1 54 94 Regorafenib (BAY 73-4506) 1 55 24 Thalidomide 1 56 40 Tivozanib (AV-951) 1 57 86 Fludarabine -

Drug Tariff Part VIIIA December 2019
12/2019 Part VIIIA BASIC PRICES OF DRUGS Drug Quantity Basic Price Category Part VIIIA - Basic Prices of Drugs Product List Part VIIIA products A Abacavir 600mg / Lamivudine 30 19000 C Lupin Healthcare 300mg tablets (UK) Ltd Abatacept 125mg/1ml solution for 4 120960 C Orencia ClickJect injection pre-filled disposable devices Abatacept 125mg/1ml solution for 4 120960 C Orencia injection pre-filled syringes Acacia spray dried powder 250 g 1632 C J M Loveridge Ltd Acamprosate 333mg gastro- 168 3770 K A resistant tablets Acarbose 100mg tablets 90 2529 A Acarbose 50mg tablets 90 1458 A Acebutolol 100mg capsules 84 1497 C Sectral Acebutolol 200mg capsules 56 1918 C Sectral Acebutolol 400mg tablets 28 1862 C Sectral Aceclofenac 100mg tablets 60 962 A Acenocoumarol 1mg tablets 100 462 C Sinthrome Acetazolamide 250mg modified- 30 1666 C Diamox SR release capsules Acetazolamide 250mg tablets 112 1029 M Acetic acid 2% ear spray S 5ml 410 C EarCalm Acetone liquid 50 ml 112 A Acetylcysteine 200mg oral powder 30 11250 A sachets sugar free Acetylcysteine 2g/10ml solution for 10 2126 C Martindale infusion ampoules Pharmaceuticals Ltd Acetylcysteine 5% eye drops S 10 ml 3346 C Ilube Acetylcysteine 600mg capsules 30 3998 A Acetylcysteine 600mg effervescent 30 550 C tablets sugar free Aciclovir 200mg dispersible tablets 25 147 A Aciclovir 200mg tablets 25 133 M Aciclovir 200mg/5ml oral 125 ml 3578 A suspension sugar free Aciclovir 3% eye ointment S 4.5 g 934 C Zovirax Aciclovir 400mg dispersible tablets 56 1199 A Aciclovir 400mg tablets 56 310 M Aciclovir -

Placebo-Controlled Trials of Chinese Herbal Medicine and Conventional Medicine: Comparative
Placebo-controlled trials of Chinese Herbal Medicine and conventional medicine: Comparative study (Aijing Shang et al.) Appendix 2 – List of matched trials of conventional medicine (1) Ala-Houhala M, Koskinen T, Koskinen M, Visakorpi JK. Double Blind Study on the Need for Vitamin D Supplementation in Prepubertal Children. Acta Paediatr Scand 1988; 77(1):89-93. (2) Arienti V, Corazza GR, Sorge M, Boriani L, Ugenti F, Biagi F et al. The effects of levosulpiride on gastric and gall-bladder emptying in functional dyspepsia. Aliment Pharmacol Ther 1994; 8(6):631-638. (3) Avner DL, Dorsch ER, Jennings DE, Greski-Rose PA. A comparison of three doses of lansoprazole (15, 30 and 60 mg) and placebo in the treatment of duodenal ulcer. Aliment Pharmacol Ther 1995; 9(5):521-528. (4) Baelde Y, Dupont P. Cetirizine in Children with Chronic Allergic Rhinitis. A Multicentre Double- Blind Study of Two Doses of Cetirizine and Placebo. Drug Invest 1992; 4(6):466-472. (5) Barabino A, Galbariggi G, Pizzorni C, Lotti G. Comparative Effects of Long-Term Therapy with Captopril and Ibopamine in Chronic Congestive Heart Failure in Old Patients. Cardiology 1991; 78(3):243-256. (6) Barbier JP, Dorf G, Gordin J, Krainik F, Neveu D, Parlier H et al. Effet de l'association métiodure de buzépide-halopéridol dans le traitement des troubles fonctionnels intestinaux. Etude randomisée en double-aveugle contrôlée contre placebo. Ann de Gastroenterologie et d'Hepatologie 1989; 25(3):123-128. (7) Benedikter L, Deichsel G, Maurer HJ. Persumbran in der Praxis. Plazebokontrollierte Doppelblindprüfung bei Patienten mit Angina pectoris. -

Preferred Drug List
Kansas State Employee ANALGESICS Second Generation cefprozil Health Plan NSAIDs cefuroxime axetil diclofenac sodium delayed-rel Preferred Drug List diflunisal Third Generation etodolac cefdinir 2021 ibuprofen cefixime (SUPRAX) meloxicam nabumetone Erythromycins/Macrolides naproxen sodium tabs azithromycin naproxen tabs clarithromycin oxaprozin clarithromycin ext-rel sulindac erythromycin delayed-rel erythromycin ethylsuccinate NSAIDs, COMBINATIONS erythromycin stearate diclofenac sodium delayed-rel/misoprostol fidaxomicin (DIFICID) Effective 04/01/2021 NSAIDs, TOPICAL Fluoroquinolones diclofenac sodium gel 1% ciprofloxacin For questions or additional information, diclofenac sodium soln levofloxacin access the State of Kansas website at moxifloxacin http://www.kdheks.gov/hcf/sehp or call COX-2 INHIBITORS Penicillins the Kansas State Employees Prescription celecoxib amoxicillin Drug Program at 1-800-294-6324. amoxicillin/clavulanate The Preferred Drug List is subject to change. GOUT amoxicillin/clavulanate ext-rel To locate covered prescriptions online, allopurinol ampicillin access the State of Kansas website at colchicine tabs dicloxacillin http://www.kdheks.gov/hcf/sehp for the probenecid penicillin VK most current drug list. colchicine (MITIGARE) Tetracyclines What is a Preferred Drug List? OPIOID ANALGESICS doxycycline hyclate A Preferred Drug List is a list of safe and buprenorphine transdermal minocycline cost-effective drugs, chosen by a committee codeine/acetaminophen tetracycline of physicians and pharmacists. Drug lists fentanyl -
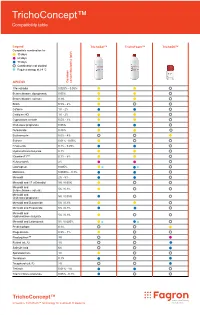
Trichoconcept™ Compatibility Table
TrichoConcept™ Compatibility table Legend TrichoSol™ TrichoFoam™ TrichoOil™ Compatible combination for 30 days 60 days 90 days Combination not studied Requires storage at 2-8 °C API/DCI Common concentration(s) (w/v) 17α-estradiol 0.025% - 0.05% Betamethasone dipropionate 0.05% Betamethasone valerate 0.1% Biotin 0.5% - 2% Caffeine 1% - 2% Cetirizine HCl 1% - 2% Cyproterone acetate 0.5% - 1% Clobetasol propionate 0.05% Dutasteride 0.25% Erythromycin 0.5% - 4% Estrone 0.01% - 0.05% Finasteride 0.1% - 0.25% Hydrocortisone butyrate 0.1% IGrantineF1™ 0.1% - 5% Ketoconazole 2% Latanoprost 0.005% Melatonin 0.0033% - 0.1% Minoxidil 2% - 5% Minoxidil and 17-α Estradiol 5% / 0.05% Minoxidil and 5% / 0.1% Betamethasone valerate Minoxidil and 5% / 0.05% Clobetasol propionate Minoxidil and Dutasteride 5% / 0.1% Minoxidil and Finasteride 5% / 0.1% Minoxidil and 5% / 0.1% Hydrocortisone butyrate Minoxidil and Latanoprost 5% / 0.005% Prednicarbate 0.1% Progesterone 0.5% - 1% Prostaquinon™ 3% Retinol (vit. A) 1% Salicylic Acid 6% Spironolactone 1% Tacrolimus 0.1% Tocopherol (vit. E) 2% Tretinoin 0.01% - 1% Triamcinolone acetonide 0.05% - 0.1% TrichoConcept™ Innovative TrichoTech™ technology for treatment of alopecia Fagron Hellas 12 km N.R. Trikala - Larisa T +30 24310 83633-5 P.C. 42100, P.O. Box 32 F +30 24310 83615 Trikala, Greece www.fagron.gr TRICHOCT02 herein. contained information or formulations the for case any in liable or responsible held be cannot and accept not does Fagron judgement. and table cannot be construed as (medical) advice, recommendation or opinion. Medical professionals, doctors and compounding pharmacists using this information are advised to do so solely if appropriate in their own professional opinion opinion professional own their in appropriate if solely so do to advised are information this using pharmacists compounding and doctors professionals, Medical opinion.