Annual Report 2020 PDF Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Artificial Intelligence in Health Care: the Hope, the Hype, the Promise, the Peril
Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril Michael Matheny, Sonoo Thadaney Israni, Mahnoor Ahmed, and Danielle Whicher, Editors WASHINGTON, DC NAM.EDU PREPUBLICATION COPY - Uncorrected Proofs NATIONAL ACADEMY OF MEDICINE • 500 Fifth Street, NW • WASHINGTON, DC 20001 NOTICE: This publication has undergone peer review according to procedures established by the National Academy of Medicine (NAM). Publication by the NAM worthy of public attention, but does not constitute endorsement of conclusions and recommendationssignifies that it is the by productthe NAM. of The a carefully views presented considered in processthis publication and is a contributionare those of individual contributors and do not represent formal consensus positions of the authors’ organizations; the NAM; or the National Academies of Sciences, Engineering, and Medicine. Library of Congress Cataloging-in-Publication Data to Come Copyright 2019 by the National Academy of Sciences. All rights reserved. Printed in the United States of America. Suggested citation: Matheny, M., S. Thadaney Israni, M. Ahmed, and D. Whicher, Editors. 2019. Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril. NAM Special Publication. Washington, DC: National Academy of Medicine. PREPUBLICATION COPY - Uncorrected Proofs “Knowing is not enough; we must apply. Willing is not enough; we must do.” --GOETHE PREPUBLICATION COPY - Uncorrected Proofs ABOUT THE NATIONAL ACADEMY OF MEDICINE The National Academy of Medicine is one of three Academies constituting the Nation- al Academies of Sciences, Engineering, and Medicine (the National Academies). The Na- tional Academies provide independent, objective analysis and advice to the nation and conduct other activities to solve complex problems and inform public policy decisions. -

Don't Risk It! ISO 13485:2016 and How to Determine Compliance Level
Don’t Risk It! ISO 13485:2016 and How to Determine Compliance Level August 18, 2016 John Beasley, RAC(US) MedTech Review, LLC Thought of the Day: “Act after having made assessments. The one who first knows the measure of far and near wins.” - Sun Tzu, The Art of War 2 Today’s Topics • Overview of changes in ISO 13485, 3rd Edition – Risk management – Usability – Outsourced processes • Supplier evaluation – determining LSP level of compliance • Concluding remarks • Q&A ISO 13485:2016 1 Overview of Changes in the 3rd Edition History of ISO 13485 13485 13485 13485 9001 9001 9001 1994 / 2000 / 2003 2015 / 1996 2008 / 2009 2016 5 Publication History 1996 - Publication of standards ISO 13485 and ISO 13488. • 13485 variant intended for manufacturers responsible for design, manufacture and distribution of devices. • 13488 variant intended for manufacturers not responsible for designing devices. Variants must be used with ISO 9001:1994 and ISO 9002:1994 as applicable. 2003 - Publication of 2nd edition ISO 13485:2003. Considered to be “stand alone”. ISO 13488 now obsolete. Organizations that previously used 13488 must now use 13485 but can exclude section 7.3 “design and development” if allowed by regulations. Device specific requirements that do not apply to the manufacturer are tagged as “not applicable”. 6 Publication History 2004 - Publication of guidance document ISO/TR 14969. Its purpose is to provide a single source of guidance on the interpretation and implementation of 13485. 2009 - Corrigendum to 13485:2003 published to change reference from ISO 9001:2000 to ISO 9001:2008. 2012 - CEN (European standards organization) publishes EN ISO 13485:2012 as the European adoption of ISO 13485:2003 (2009 corrigendum). -

Kintetsu Group Holdings Co., Ltd. 6-1-55, Uehommachi, Tennoji-Ku, Osaka-Shi, Osaka, Japan
This document has been translated from the Japanese original for the convenience of non-Japanese shareholders. In the event of any discrepancy between this translation and the Japanese original, the original shall prevail. Securities identification code: 9041 June 1, 2017 To our shareholders: Yoshinori Yoshida President Kintetsu Group Holdings Co., Ltd. 6-1-55, Uehommachi, Tennoji-ku, Osaka-shi, Osaka, Japan NOTICE OF THE 106TH ORDINARY GENERAL MEETING OF SHAREHOLDERS You are cordially invited to attend the 106th Ordinary General Meeting of Shareholders of Kintetsu Group Holdings Co., Ltd. (the “Company”), which will be held as described below. If you are unable to attend the meeting, you can exercise your voting rights in writing or via electromagnetic means (the Internet and others). Please review the Reference Documents for the General Meeting of Shareholders (from page 5 to page 17) and the Information on Exercise of Voting Rights (on page 3 and page 4) and exercise your voting rights by 6:00 p.m. on Wednesday, June 21, 2017 (Japan Standard Time). Meeting Details 1. Date and Time: Thursday, June 22, 2017 at 10:00 a.m. (Japan Standard Time) 2. Venue: 6-1-55, Uehommachi, Tennoji-ku, Osaka-shi, Osaka, Japan Sheraton Miyako Hotel Osaka, 4F “Naniwa” 3. Purposes: Items to be reported: Business Report, Consolidated Financial Statements and Non-Consolidated Financial Statements for the 106th Term (from April 1, 2016 to March 31, 2017), as well as the results of audit of the Consolidated Financial Statements by the Accounting Auditor and the Board of Auditors Items to be resolved: Proposal 1: Dividends of surplus Proposal 2: Consolidation of shares Proposal 3: Election of seventeen (17) Directors 4. -

“Global Top 10 Solution Partner”
Kintetsu World Express ANNUAL REPORT 2019 Annual Report 2019 Kintetsu World Express, Inc. Year Ended March 31, 2019 “Global Top 10 Solution Partner” A Global Brand Born in Japan Corporate Philosophy KWE Group Corporate Guidelines Contribute to the development of a global (1) We strive to further increase corporate value by (4) We are committed to comply with external delivering customers quality services that meet their regulations, and compliance monitoring and community through logistics services – needs and earn their confidence. assessment are built into all levels of the business. by creating new values, sustaining the (2) We strive to be an organization that grows and (5) We ensure a safe and healthy work environment environment and collaborating with our expands through logistics business. where people are treated respectfully and fairly. (3) We promote communications with stakeholders clients, shareholders and employees. (6) We contribute in sustainable community and disclose corporate information accurately and development, with attention to global appropriately. environmental issues. Contents 02 To Become a “Global Top 10 Solution 16 Report by Six Segments Partner” 22 ESG Section 35 Management’s Discussion and Analysis 06 Foundation for Creating New Value 40 Financial Highlights 08 Top Message 41 Financial Statements 59 Investor Information Expectations and Forecasts This annual report contains statements about our expectations and forecasts regarding plans, strategies, and business results related to the future of Kintetsu World Express, Inc. (KWE). These statements reflect our expectations based on personal beliefs and assumptions that were determined Guide to Buttons in light of information that was available at the time the report was prepared. -

Regulatory Feasibility Analysis for a New Atrial Fibrillation Ablator in Selected Countries
MQP – FRH – FHO2 Regulatory Feasibility Analysis for a New Atrial Fibrillation Ablator in Selected Countries A Major Qualifying Project Report Submitted to the Faculty of the Worcester Polytechnic Institute in partial fulfillment of the requirements for the Degree of Bachelor of Science in Management by ______________________ Matthew Hammond Approved: _______________________ _______________________ Martin Sklar, President and CEO Frank Hoy, Ph.D. AblaCor Medical Corporation Paul R. Beeswick Professor of Project Sponsor Innovation and Entrepreneurship Project Advisor _______________________ Jerome Schaufeld, Ph.D. Professor of Practice Project Co-Advisor 1 | P a g e Abstract Medical device registration for class three devices, such as a catheter ablator, can be a long and difficult process. The regulatory requirements can vary greatly by country, overlap and benefit one another, or require repeating a certification depending on the country in question. Determining the order in which to enter specific countries greatly depends on the size of the potential market and the costs and time needed for regulatory approval. Prior approval on major facets such as clinical trials may greatly reduce the costs of entering a particular country if the data from an outside source is deemed acceptable. This creates a complex problem where start- ups who cannot afford to pursue regulatory approval in all major markets simultaneously must carefully chose and enter individual markets a few at a time. The paper will evaluate the regulatory pathways of ten select countries. This regulatory analysis, combined with a market analysis on these same countries by the other three members of the project, will form a basis from which we can create a suggested order of entry. -

Risk Management for Manufacturers of in Vitro Diagnostic Medical Devices
Technical Guidance Series (TGS) Risk management for manufacturers of in 1 TGS–07 vitro diagnostic medical devices Draft for comment 25 September 2017 © World Health Organization 2017 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

FDARA Section 710 Report on the Quality, Safety, and Effectiveness of Servicing of Medical Devices
May 2018 FDA Report on the Quality, Safety, and Effectiveness of Servicing of Medical Devices In accordance with Section 710 of the Food and Drug Administration Reauthorization Act of 2017 (FDARA) Executive Summary The Food and Drug Administration Reauthorization Act (FDARA) became law on August 18, 2017. Section 710 of FDARA charges the Secretary of Health and Human Services, acting through the Commissioner of Food and Drugs, to issue a report on the continued quality, safety, and effectiveness of medical devices with respect to servicing. FDA has considered information including but not limited to the information presented at a public workshop, responses to a request for comments, and evaluation of objective evidence related to the quality, safety, and effectiveness of medical device servicing in the compilation of this report. Stakeholders have differing views about the quality, safety, and effectiveness of servicing performed by original equipment manufacturers (OEMs) and third party entities, and the need for imposing additional regulation. Based on the available information, we have concluded: • The currently available objective evidence is not sufficient to conclude whether or not there is a widespread public health concern related to servicing, including by third party servicers, of medical devices that would justify imposing additional/different, burdensome regulatory requirements at this time; • Rather, the objective evidence indicates that many OEMs and third party entities provide high quality, safe, and effective servicing of medical devices; • A majority of comments, complaints, and adverse event reports alleging that inadequate “servicing” caused or contributed to clinical adverse events and deaths actually pertain to “remanufacturing” and not “servicing”; and • The continued availability of third party entities to service and repair medical devices is critical to the functioning of the U.S. -

Phihong Ev Chargers 2021-2022
2021-2022 PHIHONG EV CHARGERS World Class Quality, International Standards Phihong Technology Co., Ltd is a core member of the organization Charging Interface Initiative e. V. (CharIN e. V.) and member of CHAdeMO Association. The goal is to promote and continuously develop the Combined Charging System (CCS) also ensuring compatibility between the infrastructure and the EVs. PHTV2102E Phihong Technology Phihong is a leading global power products manufacturer with over 50 years of industry experience. As a supplier to many of the world’s leading brands, Phihong continues to design innovative products with an emphasis on envi- ronmental protection and carbon reduction. Phihong offers a complete product line of EV charging OEM/ODM - AC Chargers solutions supporting both commercial and passenger electric vehicles. This includes Level 3 DC chargers ranging from 30kW to 360kW and Level 2 AC EVSE rang- ing from 16 to 48 amps. In addition, Phihong offers DC charging modules, auxiliary power, control & supervisor units (CSU), discrete type DC chargers, integrated type DC chargers, moveable DC chargers, and portable DC chargers. Phihongs EV charging software solutions include a front- OEM/ODM - DC Chargers end mobile APP and user interface (HMI) and a cloud- based management, payment, and monitoring platform. Through the front-end mobile APP, people can search for nearby chargers, schedule charging appointments, and monitor charging status. System operators can monitor the status of individual EV chargers and remotely up- date them, enabling long-term maintenance and man- agement. With strong R&D design capabilities and solid manufacturing experience, Phihong Technology delivers high-quality and cost-effective hardware/software prod- Modules ucts based on specific customer needs. -

Kintetsu Group Holdings
KINTETSU GROUP HOLDINGS COMPANY PROFILE 6-1-55 Uehommachi, Tennoji-ku, Osaka 543-8585, Japan https://www.kintetsu-g-hd.co.jp 2020.06 14 CULTURAL PROGRAMS SURE HOTEL and LEI To quickly respond to changes in the business environment and achieve further growth, Other under the name“Kintetsu Group Holdings Co.,Ltd.” our group companies will go all-out Businesses to satisfy all of our customers, in transportation, real estate, merchandise sales, hotel and leisure and other various businesses relating to people’s everyday life. At the same time, all group companies will work in synergy to display the collective strength of the entire Kintetsu Group. We will continue our sincere efforts to support and enrich our customers’ daily life by providing them with new delights and prosperity, thereby contributing to society’s development. Real Estate Business Hotel and Leisure Business Merchandise Sales Business Transportation Business 01 02 TRANSPORTATION TRANSPORTATION Railway Business TRANSPORTATION Kintetsu Osaka-Abenobashi Station (West gate) Iga Railway Kintetsu Group operates several railways, including Kintetsu Railway, which runs on a total track length of approximately 500 km. Covering six prefectures in the Kinki and Tokai regions, we offer interurban, sightseeing and regional transportation With our well-developed transportation services for commuters, tourists and other passengers. We constantly strive to meet REAL ESTATE customers’ various needs by improving our operation safety level, expanding barrier-free facilities, and promoting the IC ticketing system, to provide safe and comfortable network and capacity, we connect towns transportation services. with towns and people with people. Bus and Taxi Business Kintetsu Bus Kintetsu Taxi MERCHANDISE SALES We manage bus and taxi companies in the Kinki, Tokai, Hokuriku, Chugoku, Shikoku, and Kyushu regions. -

US FDA System Regulation Vs. ISO 13485:2016 Quality
US FDA QUALITY SYSTEM REGULATION VERSUS ISO 13485:2016 QUALITY MANAGEMENT SYSTEM REQUIREMENTS NSF INTERNATIONAL 21 CFR § 820 & ISO 13485:2016 ALIGNMENT CHART Author: Robert Ruff, Executive Director, NSF International Researcher: Samuel Brown, Research Associate, NSF International This tool clarifies the corresponding relationships between the US FDA Quality System Regulation and ISO 13485:2016 – Medical devices – Quality management systems – Requirements for regulatory purposes clauses. Use this tool to ensure your quality management system meets applicable requirements of both US FDA and ISO 13485:2016 21 CFR § 820 US FDA QUALITY SYSTEM REGULATION ISO 13485:2016 SPECIFIC DIFFERENCES 820.1 Scope 1 Scope Each manufacturer shall establish and 4.1.1 Quality maintain a quality system that is appropriate 820.5 Quality management for the specific medical device(s) designed No significant difference in requirements. System system, General or manufactured, and that meets the requirements requirements of this part. Management with executive responsibility shall establish its policy and objectives for, ISO 13485:2016 specifies additional detail and commitment to, quality. Management 5.3 Quality Policy 820.20(a) relative to quality policy. For example: 5.3 b), with executive responsibility shall ensure Quality Policy 5.4.1 Quality 5.3 e). ISO 13485:2016 specifically requires that the quality policy is understood, Objectives the quality objectives to be measurable. implemented, and maintained at all levels of the organization. Each manufacturer shall establish and maintain an adequate organizational structure 5.5.1 820.20(b) to ensure that devices are designed and Responsibility and No significant difference in requirements. Organization produced in accordance with the requirements Authority of this part. -
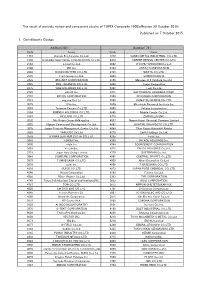
Published on 7 October 2015 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 30 October 2015) Published on 7 October 2015 1. Constituents Change Addition( 80 ) Deletion( 72 ) Code Issue Code Issue 1712 Daiseki Eco.Solution Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 1930 HOKURIKU ELECTRICAL CONSTRUCTION CO.,LTD. 2410 CAREER DESIGN CENTER CO.,LTD. 2183 Linical Co.,Ltd. 2692 ITOCHU-SHOKUHIN Co.,Ltd. 2198 IKK Inc. 2733 ARATA CORPORATION 2266 ROKKO BUTTER CO.,LTD. 2735 WATTS CO.,LTD. 2372 I'rom Group Co.,Ltd. 3004 SHINYEI KAISHA 2428 WELLNET CORPORATION 3159 Maruzen CHI Holdings Co.,Ltd. 2445 SRG TAKAMIYA CO.,LTD. 3204 Toabo Corporation 2475 WDB HOLDINGS CO.,LTD. 3361 Toell Co.,Ltd. 2729 JALUX Inc. 3371 SOFTCREATE HOLDINGS CORP. 2767 FIELDS CORPORATION 3396 FELISSIMO CORPORATION 2931 euglena Co.,Ltd. 3580 KOMATSU SEIREN CO.,LTD. 3079 DVx Inc. 3636 Mitsubishi Research Institute,Inc. 3093 Treasure Factory Co.,LTD. 3639 Voltage Incorporation 3194 KIRINDO HOLDINGS CO.,LTD. 3669 Mobile Create Co.,Ltd. 3197 SKYLARK CO.,LTD 3770 ZAPPALLAS,INC. 3232 Mie Kotsu Group Holdings,Inc. 4007 Nippon Kasei Chemical Company Limited 3252 Nippon Commercial Development Co.,Ltd. 4097 KOATSU GAS KOGYO CO.,LTD. 3276 Japan Property Management Center Co.,Ltd. 4098 Titan Kogyo Kabushiki Kaisha 3385 YAKUODO.Co.,Ltd. 4275 Carlit Holdings Co.,Ltd. 3553 KYOWA LEATHER CLOTH CO.,LTD. 4295 Faith, Inc. 3649 FINDEX Inc. 4326 INTAGE HOLDINGS Inc. 3660 istyle Inc. 4344 SOURCENEXT CORPORATION 3681 V-cube,Inc. 4671 FALCO HOLDINGS Co.,Ltd. 3751 Japan Asia Group Limited 4779 SOFTBRAIN Co.,Ltd. 3844 COMTURE CORPORATION 4801 CENTRAL SPORTS Co.,LTD. -

ISO 13485 Operational Procedure QOP-73-02 (A) Design Risk
QOP-73-02 Design Risk Management Issued by: Quality Assurance Effective Date: 6/6/2011 Rev. A Pg. 1 of 4 Approved: 6/6/2011 5:48 PM - Alan Halko, Quality Manager Risk management/analysis requirements in ISO 13485 Clause 7.3 Design Control and in 21 CFR 820.30 Design Controls are rather weak and indefinite. However, national regulatory interpretations and guidelines clearly (and strongly) clarify that risk analysis must be used in the design of medical devices. ‘ISO 14971, Medical Devices - Application of Risk Management to Medical Devices’ is most frequently cited as a reference, but full compliance with ISO 14971 is generally not required. This procedure together with ISOXpress ISO 14971 Risk Management Software respond to these regulatory guidelines for conducting risk analysis studies in design of medical devices. They generally comply with ISO 14971 Clause 4 Risk Analysis, Clause 5 Risk Evaluation, and Clause 6 Risk Control. However, note that the procedure and the software don’t address the broader ISO 14971 requirements for the overall life cycle risk management (which is normally not expected, unless full compliance with the whole ISO 14971 is explicitly required). I PURPOSE The purpose of this procedure is to provide for a system and instructions, and to assign responsibilities for conducting risk analysis studies in design of medical devices. II APPLICATION This procedure applies to the design and development of new medical devices, including their packaging and labeling, and to existing device modifications and upgrades. III PROCEDURE 1 Responsibilities 1.2 Chief Engineer is responsible for planning risk management activities, determining what risk analysis studies shall be conducted, and at which design stages; for assigning personnel (or external consultants) to conduct the studies; for evaluating the results of the studies; and for implementing any risk reduction measures recommended by the studies.