PDF-Document
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
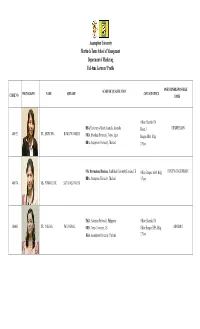
Assumption University Martin De Tours School of Management Department of Marketing Full-Time Lecturers' Profile
Assumption University Martin de Tours School of Management Department of Marketing Full-time Lecturers' Profile ACADEMIC QUALIFICATION POSITION/RESPONSIBLE PHOTOGRAPH NAME SURNAME CONTACT OFFICE CODE NO UNITS Office; Huamak: D6 DBA, University of South Australia, Australia Room 3 CHAIRPERSON 400192 DR. SUWANNA KOWATHANAKUL MBA, Shenshuu University, Tokyo, Japan Bangna: BBA Bldg. BBA, Assumption University, Thailand 2 Floor. MSc International Business, South Bank University,London, UK Office; Bangna: BBA Bldg. DEPUTY CHAIRPERSON BBA, Assumption University, Thailand 3 Floor. 400174 MS. PUNNALUCK SATANASAVAPAK Ph.D., Adamson University, Philippines Office; Huamak: D6 300005 DR. YOKFAR PHUNGPHOL MBA, Texas University, US Office Bangna: BBA Bldg. ADVISING BBA, Assumption University, Thailand 2 Floor. Assumption University Martin de Tours School of Management Department of Marketing Full-time Lecturers' Profile ACADEMIC QUALIFICATION POSITION/RESPONSIBLE PHOTOGRAPH NAME SURNAME CONTACT OFFICE CODE NO UNITS Office; Bangna: BBA Bldg. Ph.D., University of Western Australia, Perth RESEARCH & CASE STUDY 3 Floor. 380040 DR. THEINGI MBA, Assumption University, Thailand BBA, Assumption University, Thailand Office; Bangna: BBA Bldg. RESEARCH & CASE STUDY 3 Floor. 410133 Asst.Prof.Dr.Vkinda PORNSAKULVANICH Ph.D., Kent State University, US ,University of Dayton,MSA BA, Thammasat University, Thailand Ph.D. Candidate, University of Wisconsin-Milwaukee, USA Office; Bangna: BBA Bldg. 500035 MR. NITIPAN RATANASAWETWAD MS, University of Wisconsin-Milwaukee, USA 3 Floor. STUDENT ACTIVITIES/ BBA, Chulalongkorn University, Thailand INTERNAL ACTIVITIES MBA, Assumption University, Thailand Office; Bangna: BBA Bldg. STUDENT ACTIVITIES/ 370085 MS. CHO MON AUNG BBA, Assumption University, Thailand 3 Floor. INTERNAL ACTIVITIES Assumption University Martin de Tours School of Management Department of Marketing Full-time Lecturers' Profile ACADEMIC QUALIFICATION POSITION/RESPONSIBLE PHOTOGRAPH NAME SURNAME CONTACT OFFICE CODE NO UNITS MBA, Assumption University, Thailand Office; Bangna: BBA Bldg. -

Kasetsart University in Thailand: an Analysis of Institutional Evolution and Developntent Lntpact
A.LO. Project Impact Evaluation Report No. 69 Kasetsart University in Thailand: An Analysis of Institutional Evolution and Developntent lntpact September 1988 Agency for International Development (A.l.D.) Washington, D.C. 20523 PN-AAX-207 This report and others in the evaluation publication series of the Center for Development Information and Evaluation (CDIE) may be ordered from A.I.D. Document and Information Handling Facility 7222 47th Street, Suite 100 Chevy Chase, MD 20815 telephone: (301) 951-9647 A list of all CDIE evaluation publications is available from PPC/CDIE Room 105, SA-18 Agency for International Development Washington, D.C. 20523 U.S.A. telephone: (703) 875-4818 KASETSART UNIVERSITY IN THAILAND: AN ANALYSIS OF INSTITUTIONAL EVOLUTION AND DEVELOPMENT IMPACT A.I.D. PROJECT IMPACT EVALUATION REPORT NO. 69 by J.H. Eriksen, Team Leader/Agricultural Economist (Ithaca International Limited) J.L. Compton, Agricultural Extension Specialist (University of Wisconsin) N.M. Konnerup, Veterinarian (Consultant) H.D. Thurston, Plant Pathologist (Cornell University) G. Armstrong, Economist (Agency for International Development) U.S. Agency for International Development September 1988 The views and interpretations expressed in this report are those of the authors and should not be attributed to the Agency for International Development TABLE OF CONTENTS Foreword vii Acknowledgments viii Summary . x Glossary xiii 1 . Project Setting . 1 2 . Project Description 2 2 . 1 Direct U.S. Assistance Agency Support 3 2.2 Oregon State University Assistance . 3 2 . 3 Renewed Direct U.S. Assistance Agency Support 5 2.4 University of Hawaii Assistance 5 3 . Direct Indicators of Kasetsart University's Impact on the Agricultural Sector in Thailand . -

Acceptances and Matriculations Classes of 2015-2018 .Indd
Acceptances and Matriculations classes of 2015-2018 Names in bold italics represent a Class of 2018 matriculation; more than one matriculation last year is noted in parenthesis Australia and Universiteit Leiden University of Ottawa Rose-Hulman Institute of Technology New Zealand Universiteit van Amsterdam University of Toronto (2) Rutgers University University of Waterloo San Jose State University Deakin University University of Windsor Santa Clara University Griffith University United Kingdom Western University Savannah College of Art and Design Monash University (2) Aberystwyth University York University School of the Art Institute of Chicago University of Melbourne Cardiff University Seattle University University of Newcastle City University of London Seton Hall University University of Queensland United States Coventry University Simmons College University of Sydney Albany College of Pharmacy Durham University Skidmore College and Health Sciences Imperial College London Sonoma State University Arizona State University (2) India King’s College London Southeast Missouri State University Babson College London School of Economics Southwestern University Jain University Baylor University and Political Science St. Edward’s University Malaviya National Institute of Bentley University Manchester Metropolitan University Stanford University (2) Technology Berklee College of Music Newcastle University Stony Brook University Motilal Nehru National Institute Boston College Nottingham Trent University Suffolk University of Technology Boston University -

Assumption University Martin De Tours School of Management and Economics Department of Marketing Full-Time Lecturers' Profile
Assumption University Martin de Tours School of Management and Economics Department of Marketing Full-time Lecturers' Profile CODE NO PHOTOGRAPH NAME SURNAME ACADEMIC QUALIFICATION CONTACT OFFICE POSITION/RESPONSIBLE UNITS Office; Huamak: D6 DBA, University of South Australia, Australia Room 3 CHAIRPERSON 400192 DR. SUWANNA KOWATHANAKUL MBA, Shenshuu University, Tokyo, Japan Bangna: MSM Bldg. BBA, Assumption University, Thailand 2 Floor. Ph.D. Candidate, Assumption University Office; Bangna: DEPUTY CHAIRPERSON 400174 MS. PUNNALUCK SATANASAVAPAK MSc International Business, South Bank MSM Bldg. 3 Floor. University,London, UK BBA, Assumption University, Thailand Ph.D., Adamson University, Philippines Office; Huamak: D6 300005 DR. YOKFAR PHUNGPHOL MBA, Texas University, US Office Bangna: ADVISING BBA, Assumption University, Thailand MSM Bldg. 2 Floor. Assumption University Martin de Tours School of Management and Economics Department of Marketing Full-time Lecturers' Profile CODE NO PHOTOGRAPH NAME SURNAME ACADEMIC QUALIFICATION CONTACT OFFICE POSITION/RESPONSIBLE UNITS 380040 DR. THEINGI Ph.D., University of Western Australia, Perth Office; Bangna: RESEARCH & CASES STUDY MBA, Assumption University, Thailand MSM Bldg. 3 Floor. BBA, Assumption University, Thailand 410133 Ph.D., Kent State University, USA Office; Bangna: ACTING DEPUTY CHAIRPERSON ASST.PROF.DR.VIKANDA PORNSAKULVANICH MA, University of Dayton, USA MSM Bldg. 3 Floor. RESEARCH & CASES STUDY BA, Thammasat University, Thailand 500035 MR. NITIPAN RATANASAWETWAD Ph.D. Candidate, University -

Roles of Kasetsart University in Promoting Agricultural Education for Sustainable Development
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Tsukuba Repository Roles of Kasetsart University in Promoting Agricultural Education for Sustainable Development 著者 Chinnasri Buncha, Chinnasri Oranutda journal or Journal of Developments in Sustainable publication title Agriculture volume 9 number 1 page range 19-28 year 2014 URL http://hdl.handle.net/2241/00125604 Journal of Developments in Sustainable Agriculture 9: 19-28 ( 2014) Roles of Kasetsart University in Promoting Agricultural Education for Sustainable Development Buncha Chinnasri1* and Oranutda Chinnasri2 1 Faculty of Agriculture, Kasetsart University, 50 Ngamwongwan Road, Chatuchak, Bangkok, 10900, Thailand 2 Department of Sociology and Anthropology, Faculty of Social Sciences, Kasetsart University, 50 Ngamwongwan Road, Chatuchak, Bangkok, 10900, Thailand Kasetsart University (KU) was the first higher education institute to offer agricultural education in Thailand. With a strong emphasis on basic and applied agricultural research aimed at feeding the Thai people and promoting their economy, KU has been the destination of choice for students across Thailand wishing to take advantage of the university’s innovative research and educational opportunities. With the increasing acceptance of sustainable agri- culture as mainstream in Thailand, KU has pledged innovation and promotion of education and research in a diverse range of areas of sustainable agriculture and development. KU’s Faculty of Agriculture offers a master’s degree program in sustainable agriculture. With growing public recognition of the program and with financial support (especially in the form of scholarships) from KU’s International Study Center, the Sustainable Agriculture Master’s Degree Program has been recruiting more students, including those from overseas. -

Faculty of Veterinary Medicine Chiang Mai University Research Interests 2017 67
SWINE CLINIC Name: Prapas Patchanee ประภาส พัชนี E-mail: [email protected] Academic position: Associate Professor Educations: Diplomate, Thai Board of Veterinary Medicine (DTBVM) LINIC Ph.D. (Veterinary Preventive Medicines), The Ohio State University, USA C D.V.M., Kasetsart University, Bangkok, Thailand WINE Research interests: Food & Safety S Epidemiology Most recent publications: 1. Kaewsutas, M., Sarikaphuti, A., Nararatwanchai, T., Sittiprapaporn, P., Patchanee, P. Electroencephalographic study of microalgae DHA omega-3 egg consumption on cognitive function. J Funct Foods. 2017; 29, 46-52. 2. Patchanee P, Tansiricharoenkul K, Buawiratlert T, Wiratsudakul A, Angchokchatchawal K, Yamsakul P, Yano T, Boonkhot P, Rojanasatien S, Tadee P. Salmonella in pork retail outlets and dissemination of its pulsotypes through pig production chain in Chiang Mai and surrounding areas, Thailand. Prev Vet Med. 2016; 1(130): 99-105. 3. Patchanee P, Boonkhot P, Kittiwan N, Tadee P, Chotinun S. Dissemination of Salmonella enterica sequence types among Asean economic community countries. Southeast Asian J Trop Med Public Health. 2015; 46(4):707-19. 4. Chotinun S, Rojanasthien S, Unger F, Tadee P, Patchanee P. Prevalence and antimicrobial resistance of Salmonella isolated from carcasses, processing facilities and the environment surrounding small scale poultry slaughterhouses in Thailand. Southeast Asian J Trop Med Public Health. 2014; 45(6):1392-400. 5. Boonkhot P, Tadee P, Yamsakul P, Pocharoen C, Chokesajjawatee N, Patchanee P. Class 1 integrons characterization and multilocus sequence typing of Salmonella spp. from swine production chains in Chiang Mai and Lamphun provinces, Thailand. Jpn J Vet Res. 2015;63(2):83-94. 6. Tadee P, Boonkhot P, Pornruangwong S, Patchanee P. -

PROGRAM (Second Version) DAY 1 (June 4, 2015) DAY 2 (June 5, 2015) DAY 3 (June 6, 2015)
PROGRAM (Second Version) DAY 1 (June 4, 2015) DAY 2 (June 5, 2015) DAY 3 (June 6, 2015) Registration 8: Registration 9:00-10:35 Sessions A1, B1 9:00-10:35 Sessions A2, B2 9:00-10:35 Sessions A3, -- 10:35-10:50 Tea Break 10:35-10:50 Tea Break 10:35-10:50 Tea Break (Day 10:50-12:00 Keynote Speech* 10:50-12:15 Sessions C2, D2 10:50-12:15 Sessions C3, D3 s 12:00-13:25 Lunch 12:15-13:25 Lunch 12:15-13:25 Lunch 1 - 3 3) (Ratchada Room on 2/F) (Ratchada Room on 2/F) (Ratchada Room on 2/F) 0 - 16: 13:25-15:00 Sessions E1, F1 13:25-15:00 Sessions E2, F2 13:25-15:00 Sessions E3, F3 00 15:00-15:15 Tea Break 15:00-15:15 Tea Break 15:00-15:15 Closing Tea Reception 15:15-16:40 Sessions G1, H1 15:15-16:40 Sessions G2, H2 * Professor Dr. Chawin Leenabanchong, Faculty of Economics, Thammasat University (Venue: Panorama I) Session A1: EMPIRICAL FINANCE & ECONOMETRICS (venue: Panorama I) Chair: Jan Podivinsky (University of Southampton) The Reaction of Japanese REIT Market to Stock Price and Interest Rate: Comparative Analysis of the Periods before and after Abenomics (b15-046) Speaker: Takayasu Ito (Meiji University) Bayesian Estimation of a New Keynesian Policy Model for a Small Open Economy (b15-054) Speaker: Bechir Bouzid (World Bank, Washington D.C.) Optimal Matching Sequence Analysis in Economics and Finance (b15-100) Speaker: Jan Podivinsky (University of Southampton) Session B1: MARKETING, CONSUMER BEHAVIOR & PSYCHOLOGY (venue: Panorama II) Chair: Napatsorn Jiraporn (State University of New York at Oswego) An Applied Research on Feelings, Sentiments and Perceptions of Consumers in a Complaining Process in the Financial Industry using a Qualitative Data Analysis. -

The Botanical Garden at Kasetsart University Laboratory School Center for Educational Research and Development
The Botanical Garden at Kasetsart University Laboratory School Center for Educational Research and Development 著者 Techakosit Somsak journal or Journal of Developments in Sustainable publication title Agriculture volume 6 number 1 page range 131-135 year 2011 URL http://hdl.handle.net/2241/113471 Journal of Developments in Sustainable Agriculture 6: 131-135 (2011) The Botanical Garden at Kasetsart University Laboratory School Center for Educational Research and Development Somsak Techakosit* Kasetsart University Laboratory School, Faculty of Education, Kasetsart University, 50 Ngamwongwan Rd, Chatuchak Bangkok 10900, Thailand Her Royal Highness Princess Maha Chakri Sirindhorn emphasized in her book "Suan Prueksasart Rong Rian"(School Botanical Garden) that "In order to educate and train children to be aware of plant conserva tions, the children should be taught and trained to appreciate the aesthetic values, the interesting aspects and the happiness of plants by studying and conserving the plants. Conventional routine teaching and training proce dure whose emphasis is on the shoulds and should-nots and the negative impact will put the children under stress, which will not benefit the country in the long run". The school board of the academic year 1995 founded the botanical garden at Kasetsart University Labo ratory School (KUS) to raise children's awareness of the importance of the plants. Students have the oppor tunity to observe a variety of plants to create awareness of plant conservation in students' hearts. Moreover, the KUS botanical garden can be an outdoor lab for many classes. Each year, the botanical garden committee, appointed by the school board, assigns seven dilferent species of trees to be planted in the school. -

Her Royal Highness Crown Princess Maha Chakri Sirindhorn Conferring Degrees on Ramkhamhaeng University Graduates
1 ISSUE 1 (January - March 2015) Her Royal Highness Crown Princess Maha Chakri Sirindhorn Conferring Degrees on Ramkhamhaeng University Graduates Representing His Majesty the King, Her Royal Highness Crown Princess Maha Chakri Sirindhorn conferred degrees to RU graduates for the academic years 2013-2014 between March 2nd and 6th at King Ramkhamhaeng the Great Auditorium. Mr. Wirat Chinwinigkul, the chairman of Ramkhamhaeng University Council, Assistant Professor Wutisak Lapcharoensap, president of RU, administrators, faculty members, university personnel, graduate representatives, student representatives, and students from Ramkhamhaeng University Demonstration School were present at the ceremony. Ramkhamhaeng University Presenting Offerings to King Ramkhamhaeng the Great on King Ramkhamhaeng the Great Day RU hosted activities honoring King Council, presided over the ceremony and Administrators, faculty members, university Ramkhamhaeng the Great on the occasion presented offerings in honor of King personnel and students attended the ceremony of “King Ramkhamhaeng the Great Day” Ramkhamhaeng the Great together with held on the grounds of the King Ramkhamhaeng on January 17th. Mr. Wirat Chinwinigkul, Assistant Professor Wutisak Lapcharoensap, the Great Statue. the chairman of Ramkhamhaeng University president of Ramkhamhaeng University. Faculty of Fine and Applied Arts Paying Respect to Teachers of Khon, Thai Classical Music and Dance The Faculty of Fine and Applied Arts, RU, hosted a Wai Kru former RU president, presided over the ceremony, together with (Paying Respect to Teachers) Ceremony for teachers of Khon, Thai Dr. Somsak Ketkaenchan, Dean of the Faculty of Fine and Applied classical music and dance. Associate Professor Rangsan Saengsook, Arts. Faculty members, university personnel, alumni, current students and a great number of students from other schools attended the ceremony on January 15th at Room 322, 3rd floor, Sukhothai Building. -

Dr. Autchara Kayan
Department of Animal Science, Faculty of Agriculture Kasetsart University, Bangkok Thailand Dr. Autchara Kayan Dr. Agr. (Molecular genetics in animal breeding), University of Bonn, Germany MS.c. (Agriculture), Chiang Mai University, Thailand BS.c. (Agriculture), Chiang Mai University, Thailand Position: Lecturer Telephone: +66819924645 E-mail: [email protected] Teaching: Principle of meat science Advanced meat science Research Interests: Meat and Food Science (Carcass, Meat and Fat Quality) Animal Molecular Genetics Publications •Jaturasitha, S., Kayan, A. and Wicke. M. 2008. Carcass and meat characteristics of male chickens between Thai indigenous compared with improved layer breeds and their crossbred. Arch. Tierz., Dummerstorf 51:3, 283-294 •Kayan, A., Uddin, M. J., Cinar, M. U., Große-Brinkhaus, C., Phatsara, C., Wimmers, K., Ponsuksili, S., Tesfaye, D., Looft, C., Juengst, H., Tholen, E. and Schellander K. 2011. Investigation on interferon alpha-inducible protein 6 (IFI6) gene as a candidate for meat and carcass quality in pig. Meat Science. 84(4): 755-760. •Kayan, A., Cinar, M. U., Uddin, M. J., Ponsukksili, S., Wimmer, K., Phatsara, C., Tesfaye, D., Tholen, E., Juengst, H. and Schellander, K. 2011. Polymorphism and expression of the porcine Tenascin C gene associated with meat and carcass quality. Meat Science. 89(1): 76-83. •Cinar, M. U., Kayan, A., Uddin, M. J., Jonas, E. S., Tesfaye, D., Phatsara, C., Ponsuksili S., Wimmers, K., Tholen, E., Looft, C., Juengst, H. and Schellander, K. 2012. Association and expression quantitative trait loci (eQTL) analysis of porcine AMBP, GC and PPP1R3B genes with meat quality traits. Molecular Biology Reports. 39(4):4809-4821. •Kayan, A., Jaturasitha, S., Chungsiriwat, P. -

Contact Address: C/O Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Kamphaeng Saen, Nakhon Pathom 73140 Thailand
Hokkaido University [Liaison Office in Thailand] Contact address: c/o Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Kamphaeng Saen, Nakhon Pathom 73140 Thailand http://www.agri.kps.ku.ac.th/En/2012/overview.php Introduction Thai Liaison Office was opened at Kasetsart University, on May 15, 2017 Partnership with Thai University Asian Institute of Technology November 4, 2008 Mahidol University November 26, 2008 Kasetsart University January 6, 2009 Chulalongkorn University July 3, 2009 King Mongkut’s University of Technology Thonburi December 24, 2013 Thammasat University January 30,2014 King Mongkut’s Institute of Technology Ladkrabang May 30, 2014 Geo-Info & Space Tech Dev Agency (GISTDA) Aug. 3, 2018 Access: approx. 80km from Bangkok Ministry of Agriculture and Cooperatives (MOAC) Aug. 3, 2018 30 km from Nakhon Pathom Thai Liaison Office provides more information and services regarding Inquiry: [email protected] Hokkaido University and its entrance examinations to potential students. Staffs at the office plan various events, negotiate with universities to establish inter-university partnerships, and support former international students in alumni association activities. The office also serves as a hub for faculty members of Hokkaido University who visit Thailand for educational and research activities. Major activities May 15, 2017 Open of Thai Liaison Office (Faculty of Agriculture at Kamphaeng Saen, Kasetsart Univ) Feb 27, 2018 Opening Ceremony of Thai Liaison Office (Miracle Grand Hotel, Bangkok) Feb 27, 2018 Agreement of Cotutelle Program between Hokkaido Univ. & Kasetsart Univ. Mar 6, 2019 Exchanging event between Hokkaido Univ & Thai Universities Academic exchanging events (Joint seminars) have been held 4 times (2015, 2016, 2017 and 2019). -

Kasetsart University, Thailand Fact Sheet for Exchange Partner Student
2020-2021 2/2020 Kasetsart University, Thailand Fact Sheet for Exchange Partner Student University Name /Address/ Kasetsart University - Main Bangkok (BangKhen) Campus 50 Ngam Wong Wan Website Road, Ladyao, Chatuchak Bangkok 10900 THAILAND http://www.ku.ac.th/english/ General Introduction about Kasetsart University (KU) was founded in 1943 as a public university and was the the university Kingdom of Thailand’s first agricultural university. KU has four campuses: the main campus is located in Bang Khen, Bangkok. All campuses offer Bachelor, Masters, and Doctorate degree programs. Office of International http://iad.intaff.ku.ac.th/wordpress/?page_id=10486 Affairs Division ‘s Website Contact detail for exchange Ms. Araya Bijaphala, Director of International Affairs Division students Email: [email protected] Tel : +66-02-942-8171-3, Fax: +66-02-942-8170 Ms. Unthika Thamnium, inbound staff Email: [email protected] Tel: +66-02-942-8858, Fax: +66-02-942-8170 Website: https://iad.intaff.ku.ac.th/wordpress/?page_id=10486 Language of Instruction • All of the classes in KU’s international/ English programs are taught in English. (English): • Other programs at KU are taught in Thai. • Some KU Faculty Departments offer occasional classes in English; see for example, Architecture, Environmental Studies. Minimum / Maximum • Min. 9 credits Max. 22 credits (Undergraduate) course load per semester • Min. 9 credits Max. 15 credits (Graduate) Language Proficiency If not a native English speaker, proof of English proficiency is required such as Requirements: TOEFL (IBT, ITP), IELTS, TOEIC, or other assessment provided by home university in the Nomination Letter. Some Faculty and Graduate level programs may have more rigorous requirements.