A Randomized Controlled Clinical Trial Comparing Belatacept With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2017/048702 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date W O 2017/048702 A l 2 3 March 2017 (23.03.2017) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 487/04 (2006.01) A61P 35/00 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/519 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) International Application Number: DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 16/05 1490 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, 13 September 2016 (13.09.201 6) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (25) Filing Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (26) Publication Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 62/218,493 14 September 2015 (14.09.2015) US (84) Designated States (unless otherwise indicated, for every 62/218,486 14 September 2015 (14.09.2015) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: INFINITY PHARMACEUTICALS, INC. -

Project Protocol
CADTH Health Technology Assessment June 2016 (Update Drugs for the management of rheumatoid May 2017) arthritis: clinical evaluation – project protocol PROSPERO Registration Number: CRD42016041498 1 Cite as: Drugs for the management of rheumatoid arthritis: clinical evaluation – project protocol. Ottawa: CADTH; 2016 Jun; updated 2017 April. This report is prepared by the Canadian Agency for Drugs and Technologies in Health (CADTH) in collaboration with the Canadian Institutes of Health Research (CIHR) Drug Safety and Effectiveness Network (DSEN). This report contains a comprehensive review of existing public literature, studies, materials, and other information and documentation (collectively the “source documentation”) available to CADTH at the time it was prepared, and its creation was guided by expert input and advice throughout its preparation. The information in this report is intended to help health care decision-makers, patients, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. The information in this report should not be used as a substitute for the application of clinical judgment in respect to the care of a particular patient or other professional judgment in any decision-making process, nor is it intended to replace professional medical advice. While CADTH has taken care in the preparation of this report to ensure that its contents are accurate, complete, and up-to-date, CADTH does not make any guarantee to that effect. CADTH is not responsible for any errors or omissions or injury, loss, or damage arising from or as a result of the use (or misuse) of any information contained in or implied by the information in this report. -

A PILOT RANDOMIZED CONTROLLED TRIAL of DE NOVO BELATACEPT-BASED IMMUNOSUPPRESSION in LUNG TRANSPLANTATION Howard J. Huang, MD Ra
A PILOT RANDOMIZED CONTROLLED TRIAL OF DE NOVO BELATACEPT-BASED IMMUNOSUPPRESSION IN LUNG TRANSPLANTATION Howard J. Huang, MD Ramsey R. Hachem, MD Study ID: IM103-387 Draft: v 6.0 Date: 8 December 2017 ! 1 TABLE OF CONTENTS Section Page 1. List of Abbreviations 3 2. Protocol Summary 4 3. Study Schematic 4 4. Key Roles 5 5. Introduction 6 6. Objectives and Purpose 7 7. Study Design and Endpoints 7 8. Study Enrollment 8 9. Study Agent 11 10. Study Procedures and Schedule 11 11. Safety Considerations 13 12. Statistical Considerations 18 13. References 19 ! 2 LIST OF ABBREVIATIONS ACR Acute Cellular Rejection AE Adverse Event AMR Antibody-Mediated Rejection ATG Anti-Thymocyte Globulin ATS American Thoracic Society BAL Bronchoalveolar Lavage BELA Belatacept BMS Bristol-Myers Squibb BOS Bronchiolitis Obliterans Syndrome CARV Community-Acquired Respiratory Virus/Viral CBC Complete Blood Counts CFR Code of Federal Regulations CIP Cellular Immunophenotyping CKD Chronic Kidney Disease CLAD Chronic Lung Allograft Dysfunction CMP Comprehensive Metabolic Panel CMV Cytomegalovirus CNI Calcineurin Inhibitor DCC Data Coordinating Center DSA Donor-Specific HLA Antibodies DSMB Data Safety and Monitoring Board EBV Epstein-Barr Virus ECLS Extra-Corporeal Life Support ECMO Extra-Corporeal Membrane Oxygenation FOB Fiberoptic Bronchoscopy GCP Good Clinical Practice HIV Human Immunodeficiency Virus HLA Human Leukocyte Antigens HSV Herpes Simplex Virus IgG Immunoglobulin G IP Investigational Product IRB Institutional Review Board IS Immunosuppression ISHLT International -

Early Description of Coronavirus 2019 Disease in Kidney Transplant Recipients in New York
RAPID COMMUNICATION www.jasn.org Early Description of Coronavirus 2019 Disease in Kidney Transplant Recipients in New York The Columbia University Kidney Transplant Program* Department of Medicine, Division of Nephrology, Columbia University Vagelos College of Physicians and Surgeons, New York, New York ABSTRACT Background The novel SARS-CoV-2 virus has caused a global pandemic of coronavirus kidney recipients (80%), with a me- disease 2019 (COVID-19). Although immunosuppressed individuals are thought to be dian time since transplant of 49 at an increased risk of severe disease, little is known about their clinical presentation, (interquartile range, 38–118; range, disease course, or outcomes. 0–232) months (Table 1). All but one Methods We report 15 kidney transplant recipients from the Columbia University patientweretakingtacrolimusatthe kidney transplant program who required hospitalization for confirmed COVID-19, time of COVID-19 diagnosis, and and describe their management, clinical course, and outcomes. most (80%) were also taking either my- cophenolate mofetil or mycophenolic Results Patients presented most often with a fever (87%) and/or cough (67%). Initial acid. Despite our status as an early ste- chest x-ray most commonly showed bilateral infiltrates, but 33% had no acute ra- roid withdrawal center for most trans- diographic findings. Patients were managed with immunosuppression reduction plants, ten patients (67%) were taking and the addition of hydroxychloroquine and azithromycin. Although 27% of our prednisone at the time of COVID-19 patients needed mechanical ventilation, over half were discharged home by the diagnosis. The underlying cause of end of follow-up. ESKD varied. Conclusions Kidney transplant recipients with COVID-19 have presentations that Patients reported symptom onset are similar to that of the general population. -

Antibody-Mediated Rejection in Kidney Transplantation
FDA Public Workshop: Antibody-mediated Rejection in Kidney Transplantation The Choice of Induction / Maintenance Immunosuppression and their Impact on Preexisting and De Novo Antibodies Millie Samaniego, MD, FACP, FASN, FAST Alan B. Leichtman Collegiate Chair of Transplant Nephrology Professor of Medicine University of Michigan Disclosures • I have nothing to disclose in relation to this presentation: • Following drugs used “off label” will be discussed: • Thymoglobulin • Campath • IVIg • Belatacept • Rituximab • Eculizumab • C1q Inhibitors TABLE 1 The Treatment of Acute Antibody-Mediated Rejection in Kidney Transplant Recipients-A Systematic Review. Roberts, Darren; Jiang, Simon; Chadban, Steven Transplantation. 94(8):775-783, October 27, 2012. DOI: 10.1097/TP.0b013e31825d1587 TABLE 1 Therapeutic agents used against DSAs in the treatment of antibody- mediated rejection and the evidence supporting their role © 2012 Lippincott Williams & Wilkins, Inc. Published by Lippincott Williams & Wilkins, Inc. 2 FIGURE 2 The Treatment of Acute Antibody-Mediated Rejection in Kidney Transplant Recipients-A Systematic Review. Roberts, Darren; Jiang, Simon; Chadban, Steven Transplantation. 94(8):775-783, October 27, 2012. DOI: 10.1097/TP.0b013e31825d1587 FIGURE 2 . Trends in the use of treatments for AMR over time on the basis of all publications identified in the systematic review. Using a gray scale, black represents the most commonly used (in the case of PP, approximately 700 patients were treated between 2007 and 2011), whereas no color (white) means that it was not used. This includes patients from any observational, treatment, or epidemiology-based study identified in the systematic review. The use of tacrolimus and mycophenolate in patients with AMR is likely to be underrepresented in this figure because in recent years, these treatments are commonly used as baseline immunosuppression for high-risk KTRs, whereas the data included here only included new treatments administered to patients in response to a diagnosis of AMR/vascular rejection. -

Lessons Learned: Early Termination of A
Lessons Learned: Early Termination of a Randomized Trial of Calcineurin Inhibitor and Corticosteroid Avoidance Using Belatacept Kenneth Newell, Emory University Aneesh Mehta, Emory University Christian Larsen, Emory University Peter G. Stock, University of California San Francisco Alton B Farris III, Emory University Shikha G. Mehta, University of Alabama Birmingham David Ikle, Rho Inc. Brian Armstrong, Rho Inc. Yvonne Morrison, National Institute of Allergy and Infectious Diseases Nancy Bridges, National Institute of Allergy and Infectious Diseases Only first 10 authors above; see publication for full author list. Journal Title: American Journal of Transplantation Volume: Volume 17, Number 10 Publisher: Wiley: 12 months | 2017-10-01, Pages 2712-2719 Type of Work: Article | Post-print: After Peer Review Publisher DOI: 10.1111/ajt.14377 Permanent URL: https://pid.emory.edu/ark:/25593/tdqdg Final published version: http://dx.doi.org/10.1111/ajt.14377 Copyright information: © 2017 The American Society of Transplantation and the American Society of Transplant Surgeons Accessed September 30, 2021 12:24 PM EDT HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Am J Transplant Manuscript Author . Author Manuscript Author manuscript; available in PMC 2018 October 01. Published in final edited form as: Am J Transplant. 2017 October ; 17(10): 2712–2719. doi:10.1111/ajt.14377. Lessons learned: Early termination of a randomized trial of calcineurin inhibitor and corticosteroid avoidance using Belatacept Kenneth A. Newell1, -
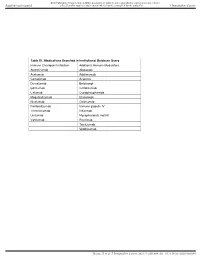
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

September 2017 ~ Resource #330909
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− September 2017 ~ Resource #330909 Medications Stored in the Refrigerator (Information below comes from current U.S. and Canadian product labeling and is current as of date of publication) Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other resources. Though most meds requiring storage at temperatures colder than room temperature should be stored in the refrigerator, expect to see a few meds require storage in the freezer. Some examples of medications requiring frozen storage conditions include: anthrax immune globulin (Anthrasil [U.S. only]), carmustine wafer (Gliadel [U.S. only]), cholera (live) vaccine (Vaxchora), dinoprostone vaginal insert (Cervidil), dinoprostone vaginal suppository (Prostin E2 [U.S.]), varicella vaccine (Varivax [U.S.]; Varivax III [Canada] can be stored in the refrigerator or freezer), zoster vaccine (Zostavax [U.S.]; Zostavax II [Canada] can be stored in the refrigerator or freezer). Use the list below to help identify medications requiring refrigerator storage and become familiar with acceptable temperature excursions from recommended storage conditions. Abbreviations: RT = room temperature Abaloparatide (Tymlos [U.S.]) Aflibercept (Eylea) Amphotericin B (Abelcet, Fungizone) • Once open, may store at RT (68°F to 77°F • May store at RT (77°F [25°C]) for up to Anakinra (Kineret) [20°C to 25°C]) for up to 30 days. -

Dimethyl Fumarate and Progressive Multifocal Leucoencephalopathy (PML)
Dimethyl fumarate and progressive multifocal leucoencephalopathy (PML) Introduction Dimethyl fumarate Psorinovo® is not registered through the Medicines Evaluation Board (MEB). It is a compounded drug made by GMP compounding pharmacy Mierlo Hout in the Netherlands, and used for the indication psoriasis [1]. Psorinovo® has been compounded by pharmacy Mierlo Hout for 28 years. According to Dutch law Mierlo Hout pharmacy is regarded as a supplying-pharmacy. ® Dimethyl fumarate, registered as Tecfidera , was granted marketing authorization in the Netherlands on 30 January 2014 and is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis [2]. Progressive multifocal leukoencephalopathy (PML) is a severe demyelinating disease of the central nervous system caused by reactivation of the polyomavirus JC (JC virus). Asymptomatic primary infection with the JC virus occurs in childhood, antibodies can be found in 86% of adults. PML occurs almost exclusively in immunosuppressed individuals. There were only isolated cases reported of PML in patients without apparent immunosuppression. However, there are reports of PML affecting patients who have conditions associated with minimal or occult immunosuppression, such as hepatic cirrhosis and renal failure [3]. PML has also been reported in patients treated with drugs such as belatacept, brentuximab, efalizumab, fludarabine, glucocorticoids, infliximab, mycophenolate, rituximab, ruxolitinib and natalizumab. In some cases, these drugs were used in combination with other immunosuppressive medications (eg, cyclophosphamide, leflunomide, methotrexate). Many of the patients had an underlying hematologic malignancy or collagen vascular disease [3]. There is no specific treatment for PML. The main approach is restoring the host adaptive immune response, a strategy that appears to prolong survival. -

Authorized Distributors Matrix
Authorized Distributors* September 1, 2021 ORENCIA® ClickJect™ KENALOG® ORENCIA® ORENCIA® (abatacept) EVOTAZ® (triamcinolone (abatacept) (abatacept) injection, Single- ABRAXANE® (atazanavir acetonide injection, for injection, for Dose Autoinjector REBLOZYL® ZEPOSIA® (paclitaxel AZACTAM® BARACLUDE® DROXIA® ELIQUIS® EMPLICITI® and HYDREA® IDHIFA® INREBIC® ISTODAX® injectable NULOJIX® ONUREG® OPDIVO® intravenous subcutaneous for subcutaneous POMALYST® (luspatercept- REVLIMID® REYATAZ® SPRYCEL® SUSTIVA® THALOMID® VIDAZA® YERVOY® (ozanimod Company protein-bound) (aztreonam) (entecavir) (hydroxyurea) (apixaban) (elotuzumab) cobicistat) (hydroxyurea) (enasidenib) (fedratinib) (romidepsin) suspension USP) (belatacept) (azacitidine) (nivolumab) use use use (pomalidomide) aamt) (lenalidomide) (atazanavir) (dasatinib) (efavirenz) (thalidomide) (azacitidine) (ipilimumab) capsules) Amerisourcebergen Corporation ••••• •• • • •• • • • •••• ASD Specialty Healthcare, Inc. • •• ••••• ••• • • • • •••• ASD Healthcare And Oncology Supply • •• ••••• ••• • • • • •••• Besse Medical •• ••• • Anda Inc. •• • • •• • •• • ••• Baxter Healthcare Corporation • Capital Wholesale Drug Co. •• • • •• • • • ••• Cardinal Health, Inc. ••••• •• • •• • • • •••• Cardinal Health Ambulatory Care • Cardinal Health Specialty Pharmaceutical Distribution • •• ••••• •••• •••• • •••• Metro Medical Supply, Inc., A Subsidiary Of Cardinal Specialty •••••• Clint Pharmaceuticals, Inc. • Dakota Drug, Inc. •• • • •• • • • ••• Dixon Shane/R&S Northeast, Llc •• • • •• • • • ••• DMS Pharmaceutical -

Dossier Zur Nutzenbewertung Gemäß § 35A SGB V
Dokumentvorlage, Version vom 20.01.2011 Dossier zur Nutzenbewertung gemäß § 35a SGB V Belatacept (NulojixTM) Bristol-Myers Squibb GmbH & Co. KG Modul 2 Allgemeine Angaben zum Arzneimittel, zugelassene Anwendungsgebiete Stand: 13.01.2012 Dossier zur Nutzenbewertung – Modul 2 Stand: 13.01.2012 Allgemeine Angaben zum Arzneimittel, zugelassene Anwendungsgebiete Inhaltsverzeichnis Seite Tabellenverzeichnis .................................................................................................................. 2 Abbildungsverzeichnis ............................................................................................................. 3 Abkürzungsverzeichnis ............................................................................................................ 4 2 Modul 2 – allgemeine Informationen ............................................................................ 5 2.1 Allgemeine Angaben zum Arzneimittel ....................................................................... 5 2.1.1 Administrative Angaben zum Arzneimittel ............................................................ 5 2.1.2 Angaben zum Wirkmechanismus des Arzneimittels ............................................... 6 2.2 Zugelassene Anwendungsgebiete ............................................................................... 12 2.2.1 Anwendungsgebiete, auf die sich das Dossier bezieht .......................................... 12 2.2.2 Weitere in Deutschland zugelassene Anwendungsgebiete ................................... 14 2.2.3 Zulassungsstatus -

SUPPLEMENTARY APPENDIX 4: Search Strategies Syntax Guide For
SUPPLEMENTARY APPENDIX 4: Search Strategies Pubmed, Embase Perioperative Management - PubMed Search Strategy – March 6, 2016 Syntax Guide for PubMed [MH] = Medical Subject Heading, also [TW] = Includes all words and numbers in known as MeSH the title, abstract, other abstract, MeSH terms, MeSH Subheadings, Publication Types, Substance Names, Personal Name as Subject, Corporate Author, Secondary Source, Comment/Correction Notes, and Other Terms - typically non-MeSH subject terms (keywords)…assigned by an organization other than NLM [SH] = a Medical Subject Heading [TIAB] = Includes words in the title and subheading, e.g. drug therapy abstracts [MH:NOEXP] = a command to retrieve the results of the Medical Subject Heading specified, but not narrower Medical Subject Heading terms Boolean Operators OR = retrieves results that include at least AND = retrieves results that include all the one of the search terms search terms NOT = excludes the retrieval of terms from the search Perioperative Management PubMed Search Strategy – March 6, 2016 Search Query #1 ((((ARTHROPLASTY, REPLACEMENT, HIP[MH] OR HIP PROSTHES*[TW] OR HIP REPLACEMENT*[TIAB] OR HIP ARTHROPLAST*[TIAB] OR HIP TOTAL REPLACEMENT*[TIAB] OR FEMORAL HEAD PROSTHES*[TIAB]) OR (ARTHROPLASTY, REPLACEMENT, KNEE[MH] OR KNEE PROSTHES*[TW] OR KNEE REPLACEMENT*[TW] OR KNEE ARTHROPLAST*[TW] OR KNEE TOTAL REPLACEMENT*[TIAB]) OR (ARTHROPLAST*[TW] AND (HIP[TIAB] OR HIPS[TIAB] OR KNEE*[TIAB])) AND (("1980/01/01"[PDAT] : "2016/03/06"[PDAT]) AND ENGLISH[LANG])) NOT (((("ADOLESCENT"[MESH]) OR "CHILD"[MESH])