SSRI Augmentation by 5-Hydroxytryptophan Slow Release: Mouse Pharmacodynamic Proof of Concept
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activation of 5-HT2C (But Not 5-HT1A) Receptors in the Amygdala Enhances Fear-Induced Antinociception: Blockade with Local 5-HT2C Antagonist Or Systemic fluoxetine
Neuropharmacology 135 (2018) 376e385 Contents lists available at ScienceDirect Neuropharmacology journal homepage: www.elsevier.com/locate/neuropharm Activation of 5-HT2C (but not 5-HT1A) receptors in the amygdala enhances fear-induced antinociception: Blockade with local 5-HT2C antagonist or systemic fluoxetine Lígia Renata Rodrigues Tavares a, b, Daniela Baptista-de-Souza a, c, * Azair Canto-de-Souza a, b, c, d, a Psychobiology Group, Department of Psychology/CECH- Federal University of Sao~ Carlos-UFSCar, Sao~ Carlos, Sao~ Paulo, 13565-905, Brazil b Joint Graduate Program in Physiological Sciences UFSCar/UNESP, Sao~ Carlos, Sao~ Paulo, 13565-905, Brazil c Neuroscience and Behavioral Institute-IneC, Ribeirao~ Preto, Sao~ Paulo, 14040-901, Brazil d Program in Psychology UFSCar, Sao~ Carlos, Sao~ Paulo, 13565-905, Brazil article info abstract Article history: It is well-known that the exposure of rodents to threatening environments [e.g., the open arm of the Received 17 August 2017 elevated-plus maze (EPM)] elicits pain inhibition. Systemic and/or intracerebral [e.g., periaqueductal gray Received in revised form matter, amygdala) injections of antiaversive drugs [e.g., serotonin (5-HT) ligands, selective serotonin 5 March 2018 reuptake inhibitors (SSRIs)] have been used to change EPM-open arm confinement induced anti- Accepted 6 March 2018 nociception (OAA). Here, we investigated (i) the role of the 5-HT and 5-HT receptors located in the Available online 13 March 2018 1A 2C amygdaloid complex on OAA as well as (ii) the effects of systemic pretreatment with fluoxetine (an SSRI) on the effects of intra-amygdala injections of 8-OH-DPAT (a 5-HT1A agonist) or MK-212 (a 5-HT2C agonist) Keywords: fi Amygdala on nociception in mice con ned to the open arm or enclosed arm of the EPM. -

Paradoxical Actions of the Serotonin Precursor 5-Hydroxytryptophan on the Activity of Identified Serotonergic Neurons in a Simple Motor Circuit
The Journal of Neuroscience, February 15, 2000, 20(4):1622–1634 Paradoxical Actions of the Serotonin Precursor 5-hydroxytryptophan on the Activity of Identified Serotonergic Neurons in a Simple Motor Circuit David J. Fickbohm and Paul S. Katz Department of Biology, Georgia State University, Atlanta, Georgia 30302 Neurotransmitter synthesis is regulated by a variety of factors, cated an increase in a putative 5-HT electrochemical signal yet the effect of altering transmitter content on the operation of during swim CPG activation. Paradoxically, the spiking activity neuronal circuits has been relatively unexplored. We used elec- of the serotonergic neurons decreased to a single burst at the trophysiological, electrochemical, and immunohistochemical onset of the rhythmic motor program, whereas the overall techniques to investigate the effects of augmenting the seroto- duration of the episode remained about the same. 5-HTP treat- nin (5-HT) content of identified serotonergic neurons embed- ment gradually reduced the rhythmicity of the CPG output. ded in a simple motor circuit. The dorsal swim interneurons Thus, more serotonin did not result in a more robust swim (DSIs) are serotonergic neurons intrinsic to the central pattern motor program, suggesting that serotonin synthesis must be generator (CPG) for swimming in the mollusc Tritonia diom- kept within certain limits for the circuit to function correctly and edea. As expected, treatment with the serotonin precursor indicating that altering neurotransmitter synthesis can have 5-hydroxytryptophan (5-HTP) increased the intensity of seroto- serious consequences for the output of neural networks. nin immunolabeling and enhanced the potency of synaptic and Key words: intrinsic neuromodulation; central pattern gener- modulatory actions elicited by the DSIs. -

(DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact
pharmaceuticals Review Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact Andreia Machado Brito-da-Costa 1 , Diana Dias-da-Silva 1,2,* , Nelson G. M. Gomes 1,3 , Ricardo Jorge Dinis-Oliveira 1,2,4,* and Áurea Madureira-Carvalho 1,3 1 Department of Sciences, IINFACTS-Institute of Research and Advanced Training in Health Sciences and Technologies, University Institute of Health Sciences (IUCS), CESPU, CRL, 4585-116 Gandra, Portugal; [email protected] (A.M.B.-d.-C.); ngomes@ff.up.pt (N.G.M.G.); [email protected] (Á.M.-C.) 2 UCIBIO-REQUIMTE, Laboratory of Toxicology, Department of Biological Sciences, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal 3 LAQV-REQUIMTE, Laboratory of Pharmacognosy, Department of Chemistry, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal 4 Department of Public Health and Forensic Sciences, and Medical Education, Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal * Correspondence: [email protected] (D.D.-d.-S.); [email protected] (R.J.D.-O.); Tel.: +351-224-157-216 (R.J.D.-O.) Received: 21 September 2020; Accepted: 20 October 2020; Published: 23 October 2020 Abstract: Ayahuasca is a hallucinogenic botanical beverage originally used by indigenous Amazonian tribes in religious ceremonies and therapeutic practices. While ethnobotanical surveys still indicate its spiritual and medicinal uses, consumption of ayahuasca has been progressively related with a recreational purpose, particularly in Western societies. The ayahuasca aqueous concoction is typically prepared from the leaves of the N,N-dimethyltryptamine (DMT)-containing Psychotria viridis, and the stem and bark of Banisteriopsis caapi, the plant source of harmala alkaloids. -

A Serotonin Receptor with a Possible Role in Joint Diseases
Anders Kling 5-HT2 A – a serotonin receptor with a possible role in joint diseases role with a possible receptor – a serotonin 5-HT2A – a serotonin receptor with a possible role in joint diseases Anders Kling Umeå University 2013 Umeå University Department of Pharmacology and Clinical Neuroscience New Serie 1547 Department of Pharmacology and Clinical Neurosciences Umeå University ISSN: 0346-6612 Umeå University, Sweden 2013 SE-901 87 Umeå, Sweden ISBN 978-91-7459-549-9 5-HT2A – a serotonin receptor with a possible role in joint diseases Anders Kling Institutionen för farmakologi och klinisk neurovetenskap, Klinisk farmakologi/ Department of Pharmacology and Clinical Neuroscience, Clinical Pharmacology Umeå universitet/ Umeå University Umeå 2013 Responsible publisher under swedish law: the Dean of the Medical Faculty This work is protected by the Swedish Copyright Legislation (Act 1960:729) ISBN: 978-91-7459-549-9 ISSN: 0346-6612 New series No: 1547 Elektronisk version tillgänglig på http://umu.diva-portal.org/ Tryck/Printed by: Print och Media, Umeå universitet Umeå, Sweden 2013 Innehåll/Table of Contents Innehåll/Table of Contents i Abstract iv Abbreviations vi List of studies viii Populärvetenskaplig sammanfattning ix 5-HT2A – en serotoninreceptor med möjlig betydelse för ledsjukdomar ix Introduction 1 The serotonin system 1 Serotonin 1 Serotonin receptors 2 The serotonin system and platelets 2 Serotonin receptor 5-HT2A 3 Localisation/expression of 5-HT2A receptors 3 Functions of the 5-HT2A receptor 4 Regulation of the 5-HT2A receptor -

Serotonin Autoreceptors on Dorsal Raphe Neurons: Structure-Activity Relationships of Tryptamine Analogs’
0270.6474/81/0110-1148$o2.oo/0 The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 1, No. 10, pp. 1148-1154 Printed in U.S.A. October 1981 SEROTONIN AUTORECEPTORS ON DORSAL RAPHE NEURONS: STRUCTURE-ACTIVITY RELATIONSHIPS OF TRYPTAMINE ANALOGS’ MICHAEL A. ROGAWSKI’ AND GEORGE K. AGHAJANIAN Departments of Pharmacology and Psychiatry, Yale University School of Medicine and the Connecticut Mental Health Center, New Haven, Connecticut 06510 Abstract A series of indole-ethylamines were tested for their ability to suppress the spontaneous firing of single dorsal raphe serotonergic neurons in the rat. The compounds were all derivatives of either tryptamine or N,N-dimethyltryptamine possessing hydroxy or methoxy substituents on the benzene ring portion of the indole nucleus. Their activity was assessed using quantitative microiontophoresis or following systemic (intravenous) administration. The serotonin autoreceptor or so-called “S2 receptor” mediating the inhibition of raphe serotonergic neurons was found to exhibit a high degree of structural specificity among the closely related tryptamine analogs. The following structure- activity rules were demonstrated: (1) for either hydroxy or methoxy derivatives, the relative favorability of the ring positions conforms to the series 5 >> 4 > 6; (2) methoxy derivatives are more sensitive to a shift of the ring substituent from the 5- to the 4- or 6-positions than are hydroxy compounds; and (3) activity is enhanced by N,N-dimethylation. Furthermore, addition of a methyl group at the 7-position of 5-methoxy-N,N-dimethyltryptamine markedly reduces the activity of this potent agonist. Of the radioligands which label brain serotonin receptors, the pharmacological characteristics of D-[“HIlysergic acid diethylamide binding best correspond to those displayed by the SZ receptor as determined in the present physiological analysis, although sufficient data are not yet available to make a complete comparison. -

Psychedelics: a Window to Mental Illness; Psilocybin and Depression
Nova Southeastern University NSUWorks College of Pharmacy Student Articles College of Pharmacy 7-22-2020 Psychedelics: A Window To Mental Illness; Psilocybin And Depression Kiomary Rivera Quintana [email protected] Follow this and additional works at: https://nsuworks.nova.edu/hpd_corx_stuarticles Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Rivera Quintana, Kiomary, "Psychedelics: A Window To Mental Illness; Psilocybin And Depression" (2020). College of Pharmacy Student Articles. 5. https://nsuworks.nova.edu/hpd_corx_stuarticles/5 This Literature Review is brought to you for free and open access by the College of Pharmacy at NSUWorks. It has been accepted for inclusion in College of Pharmacy Student Articles by an authorized administrator of NSUWorks. For more information, please contact [email protected]. 2020 Psychedelics: A Window to Mental Illness PSILOCYBIN AND DEPRESSION KIOMARY RIVERA QUINTANA STUDENT PHARMACIST CLASS OF 2022 PHRE 5223-DRUGS OF ABUSE NOVA SOUTHEASTERN UNIVERSITY INTRODUCTION Drug abuse is characterized by improper repeated use of drugs to seek outcomes such as pleasure, stress relief and an altered reality.1 It can lead to addiction, a severe form of substance use disorder (SUD) in which a person’s drug habits worsen and they become unable to control the impulse to use drugs despite knowing the negative consequences.1 In addition to drug seeking behavior, brain function also changes affecting the natural inhibition and reward centers. Use of and addiction to alcohol, nicotine, and illicit drugs costs the Nation more than $740 billion a year related to healthcare, crime, and lost productivity.1 Whether a person will abuse drugs or become addicted is influenced by multiple factors; the more risk factors a person has, the greater the chance. -

Lysergic Acid Diethylamide (LSD) Promotes Social Behavior Through Mtorc1 in the Excitatory Neurotransmission
Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission Danilo De Gregorioa,b,1, Jelena Popicb,2,3, Justine P. Ennsa,3, Antonio Inserraa,3, Agnieszka Skaleckab, Athanasios Markopoulosa, Luca Posaa, Martha Lopez-Canula, He Qianzia, Christopher K. Laffertyc, Jonathan P. Brittc, Stefano Comaia,d, Argel Aguilar-Vallese, Nahum Sonenbergb,1,4, and Gabriella Gobbia,f,1,4 aNeurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montreal, QC, Canada, H3A 1A1; bDepartment of Biochemistry, McGill University, Montreal, QC, Canada, H3A 1A3; cDepartment of Psychology, McGill University, Montreal, QC, Canada, H3A 1B1; dDivision of Neuroscience, Vita Salute San Raffaele University, 20132 Milan, Italy; eDepartment of Neuroscience, Carleton University, Ottawa, ON, Canada, K1S 5B6; and fMcGill University Health Center, Montreal, QC, Canada, H3A 1A1 Contributed by Nahum Sonenberg, November 10, 2020 (sent for review October 5, 2020; reviewed by Marc G. Caron and Mark Geyer) Clinical studies have reported that the psychedelic lysergic acid receptor (6), but also displays affinity for the 5-HT1A receptor diethylamide (LSD) enhances empathy and social behavior (SB) in (7–9). Several studies have demonstrated that LSD modulates humans, but its mechanism of action remains elusive. Using a glutamatergic neurotransmission and, indirectly, the α-amino- multidisciplinary approach including in vivo electrophysiology, 3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors. optogenetics, behavioral paradigms, and molecular biology, the Indeed, in vitro studies have shown that LSD increased the ex- effects of LSD on SB and glutamatergic neurotransmission in the citatory response of interneurons in the piriform cortex following medial prefrontal cortex (mPFC) were studied in male mice. -

Differential Regulation of Serotonin 2A Receptor Responsiveness by Agonist
Differential regulation of serotonin 2A receptor responsiveness by agonist- directed interactions with arrestin2 DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Cullen Laura Schmid, B.S. Neuroscience Graduate Studies Program The Ohio State University 2011 Dissertation Committee: Laura M. Bohn, Co-advisor Georgia A. Bishop, Co-advisor Candice C. Askwith Wolfgang Sadee Copyright by Cullen Laura Schmid 2011 Abstract The G protein-coupled, serotonin 2A (5-HT2A) receptor is a major drug target for the treatment of a number of mental health disorders, including schizophrenia, anxiety and depression. In addition to modulating several of the physiological effects of the neurotransmitter serotonin, activation of the 5-HT2A receptor mediates the psychotomimetic effects of serotonergic hallucinogenic drugs, such as lysergic acid diethylamide (LSD), 2,5-dimethoxy-4-iodoamphetamine (DOI) and 5-methoxy-N,N- dimethyltryptamine (5-MeO-DMT). Though hallucinogens are agonists at the 5-HT2A receptor, not all 5-HT2A receptor agonists induce hallucinations in humans, including the endogenous ligand serotonin. Therefore, the activation of the 5-HT2A receptor can result in different biological responses depending upon the chemical nature of the ligand, a concept that has been referred to as “functional selectivity.” One way in which ligands can induce differential signaling at GPCRs is through interactions with arrestins, which can act to dampen or facilitate receptor signaling cascades or mediate the internalization of receptors into intracellular vesicles. The overarching hypothesis of this dissertation is that the interaction between the regulatory protein, arrestin2, and the 5-HT2A receptor is a critical point in the divergence of agonist-directed 5-HT2A receptor responsiveness. -

Hallucinogens: an Update
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Hallucinogens: An Update 146 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Hallucinogens: An Update Editors: Geraline C. Lin, Ph.D. National Institute on Drug Abuse Richard A. Glennon, Ph.D. Virginia Commonwealth University NIDA Research Monograph 146 1994 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGEMENT This monograph is based on the papers from a technical review on “Hallucinogens: An Update” held on July 13-14, 1992. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company. -

N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo Jose A
Morales-Garcia et al. Translational Psychiatry (2020) 10:331 https://doi.org/10.1038/s41398-020-01011-0 Translational Psychiatry ARTICLE Open Access N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo Jose A. Morales-Garcia 1,2,3,4, Javier Calleja-Conde 5, Jose A. Lopez-Moreno 5, Sandra Alonso-Gil1,2, Marina Sanz-SanCristobal1,2, Jordi Riba6 and Ana Perez-Castillo 1,2,4 Abstract N,N-dimethyltryptamine (DMT) is a component of the ayahuasca brew traditionally used for ritual and therapeutic purposes across several South American countries. Here, we have examined, in vitro and vivo, the potential neurogenic effect of DMT. Our results demonstrate that DMT administration activates the main adult neurogenic niche, the subgranular zone of the dentate gyrus of the hippocampus, promoting newly generated neurons in the granular zone. Moreover, these mice performed better, compared to control non-treated animals, in memory tests, which suggest a functional relevance for the DMT-induced new production of neurons in the hippocampus. Interestingly, the neurogenic effect of DMT appears to involve signaling via sigma-1 receptor (S1R) activation since S1R antagonist blocked the neurogenic effect. Taken together, our results demonstrate that DMT treatment activates the subgranular neurogenic niche regulating the proliferation of neural stem cells, the migration of neuroblasts, and promoting the generation of new neurons in the hippocampus, therefore enhancing adult neurogenesis and -
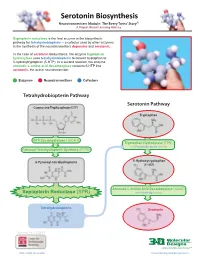
Serotonin Biosynthesis Neurotransmitters Module: the Beery Twins’ Story© a Project-Based Learning Activity
Serotonin Biosynthesis Neurotransmitters Module: The Beery Twins’ Story© A Project-Based Learning Activity Sepiapterin reductase is the final enzyme in the biosynthetic pathway for tetrahydrobiopterin – a cofactor used by other enzymes in the synthesis of the neurotransmitters dopamine and serotonin. In the case of serotonin biosynthesis, the enzyme tryptophan hydroxylase uses tetrahydrobiopterin to convert tryptophan to 5-hydroxytryptophan (5-HTP). In a second reaction, the enzyme aromatic L-amino acid decarboxylase converts 5-HTP into serotonin, the active neurotransmitter. Enzymes Neurotransmitters Cofactors Tetrahydrobiopterin Pathway Serotonin Pathway Guanosine Triphosphate (GTP) Tryptophan GTP Cyclohydrolase I (GCH1) Tryptophan Hydroxylase (TPH) with Tetrahydrobiopterin Cofactor Pyruvoyl-Tetrahydropterin Synthase (PTPS) 6-Pyruvoyl-tetrahydropterin 5-Hydroxytryptophan (5-HTP) Aromatic L-Amino Acid Decarboxylase (AAAD) Sepiapterin Reductase (SPR) with Vitamin B6 Cofactor Tetrahydrobiopterin Serotonin Version 1.4 -12/2015 ...where molecules become real TM http://cbm.msoe.edu www.3dmoleculardesigns.com Serotonin Biosynthesis Model Guide Neurotransmitters Module: The Beery Twins’ Story© A Project-Based Learning Activity TPH AAAD Tryptophan 5-Hydroxytryptophan Serotonin Tryptophan (Trp or W) is one of the 20 standard 5-Hydroxytryptophan, an intermediate The nal step in the serotonin biosynthesis amino acids and is an essential amino acid that molecule in the serotonin biosynthesis pathway requires the removal of the cannot be synthesized by the human body. pathway, is formed by the addition of a carboxylic acid group (COOH) from the Tryptophan is composed of the standard amino hydroxyl (OH) group to the fth carbon of the backbone of 5-hydroxytryptophan to form acid backbone with an indole ring side chain. indole ring of tryptophan. -

Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans
molecules Review Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans Paul Cumming 1,2,* , Milan Scheidegger 3 , Dario Dornbierer 3, Mikael Palner 4,5,6 , Boris B. Quednow 3,7 and Chantal Martin-Soelch 8 1 Department of Nuclear Medicine, Bern University Hospital, CH-3010 Bern, Switzerland 2 School of Psychology and Counselling, Queensland University of Technology, Brisbane 4059, Australia 3 Department of Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital of the University of Zurich, CH-8032 Zurich, Switzerland; [email protected] (M.S.); [email protected] (D.D.); [email protected] (B.B.Q.) 4 Odense Department of Clinical Research, University of Southern Denmark, DK-5000 Odense, Denmark; [email protected] 5 Department of Nuclear Medicine, Odense University Hospital, DK-5000 Odense, Denmark 6 Neurobiology Research Unit, Copenhagen University Hospital, DK-2100 Copenhagen, Denmark 7 Neuroscience Center Zurich, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8058 Zurich, Switzerland 8 Department of Psychology, University of Fribourg, CH-1700 Fribourg, Switzerland; [email protected] * Correspondence: [email protected] or [email protected] Abstract: Hallucinogens are a loosely defined group of compounds including LSD, N,N- dimethyltryptamines, mescaline, psilocybin/psilocin, and 2,5-dimethoxy-4-methamphetamine (DOM), Citation: Cumming, P.; Scheidegger, which can evoke intense visual and emotional experiences. We are witnessing a renaissance of re- M.; Dornbierer, D.; Palner, M.; search interest in hallucinogens, driven by increasing awareness of their psychotherapeutic potential. Quednow, B.B.; Martin-Soelch, C. As such, we now present a narrative review of the literature on hallucinogen binding in vitro and Molecular and Functional Imaging ex vivo, and the various molecular imaging studies with positron emission tomography (PET) or Studies of Psychedelic Drug Action in single photon emission computer tomography (SPECT).