Complications in the Treatment of Carpal Tunnel Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carpal Tunnel Syndrome
Information O from Your Family Doctor Carpal Tunnel Syndrome What is carpal tunnel syndrome? hands a lot. You may notice that over time your Carpal tunnel syndrome (KAR-pal TUN-el grip gets weaker and you tend to drop heavy SIN-drome) is a common, painful disorder objects. of the wrist and hand. It happens when the median nerve, which runs through the wrist, How is it diagnosed? gets squeezed under a band of tissue called a Talk to your doctor if you are having these ligament. This causes pain and other symptoms symptoms. He or she will ask questions about along the nerve (see drawing). the ways you use your hands and about specific What causes it? Anything that increases Shading indicates area pressure on the median where symptoms are felt nerve can cause carpal tunnel syndrome. Sometimes pregnancy and health conditions like arthritis and diabetes can increase the pressure. People who use their hands and wrists repeatedly in the same way (for example, Transverse typists, carpenters, and carpal cashiers) are more likely to get ligament carpal tunnel syndrome. What are the symptoms? Carpal tunnel syndrome Thenar may cause pain, numbness, muscles Wrist or tingling in your wrist and bone hand, mostly in the middle finger, index finger, and Tendon thumb. The symptoms are Median usually worse at night and nerve when you use your wrists and ILLUSTRATION BY KATHRYN BORN continued O Page 1 of 2 Information O from Your Family Doctor Carpal Tunnel Syndrome (continued) symptoms in each part of your hand and wrist. He or she may also test how your nerves and Notes: muscles respond to electrical stimulation. -
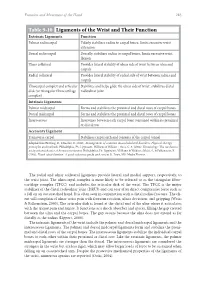
Table 9-10 Ligaments of the Wrist and Their Function
Function and Movement of the Hand 283 Table 9-10 Ligaments of the Wrist and Their Function Extrinsic Ligaments Function Palmar radiocarpal Volarly stabilizes radius to carpal bones; limits excessive wrist extension Dorsal radiocarpal Dorsally stabilizes radius to carpal bones; limits excessive wrist flexion Ulnar collateral Provides lateral stability of ulnar side of wrist between ulna and carpals Radial collateral Provides lateral stability of radial side of wrist between radius and carpals Ulnocarpal complex and articular Stabilizes and helps glide the ulnar side of wrist; stabilizes distal disk (or triangular fibrocartilage radioulnar joint complex) Intrinsic Ligaments Palmar midcarpal Forms and stabilizes the proximal and distal rows of carpal bones Dorsal midcarpal Forms and stabilizes the proximal and distal rows of carpal bones Interosseous Intervenes between each carpal bone contained within its proximal or distal row Accessory Ligament Transverse carpal Stabilizes carpal arch and contents of the carpal tunnel Adapted from Hertling, D., & Kessler, R. (2006). Management of common musculoskeletal disorders: Physical therapy principles and methods. Philadelphia, PA: Lippincott, Williams & Wilkins.; Oatis, C. A. (2004). Kinesiology: The mechanics and pathomechanics of human movement. Philadelphia, PA: Lippincott, Williams & Wilkins.; Weiss, S., & Falkenstein, N. (2005). Hand rehabilitation: A quick reference guide and review. St. Louis, MO: Mosby Elsevier. The radial and ulnar collateral ligaments provide lateral and medial support, respectively, to the wrist joint. The ulnocarpal complex is more likely to be referred to as the triangular fibro- cartilage complex (TFCC) and includes the articular disk of the wrist. The TFCC is the major stabilizer of the distal radioulnar joint (DRUJ) and can tear after direct compressive force such as a fall on an outstretched hand. -

Carpal Tunnel
CLINICAL MEDICAL POLICY Policy Name: Carpal Tunnel Syndrome Policy Number: MP-031-MC-ALL Responsible Department(s): Medical Management Provider Notice Date: 08/15/2019; 08/15/2018; 08/15/2017 Issue Date: 09/16/2019; 09/15/2018 Original Effective Date: 09/16/2019; 09/15/2018; 09/15/2017 Annual Approval Date: 07/17/2020 Revision Date: 07/17/2019; 07/18/2018 Products: Ohio Medicare Assured All participating and nonparticipating hospitals and Application: providers Page Number(s): 1 of 10 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ provides coverage under the medical-surgical benefits of the Company’s Medicare products for medically necessary carpal tunnel surgical procedures to treat carpal tunnel syndrome. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. DEFINITIONS Carpal Tunnel – A narrow, rigid passageway of ligament and bones at the base of the hand. The carpal tunnel houses the median nerve and the tendons that bend the fingers. The median nerve provides feeling to the palm side of the thumb and to the index, middle, and part of the ring fingers. The median nerve also controls some small muscles at the base of the thumb. -

Musculoskeletal Ultrasound Technical Guidelines III. Wrist
European Society of MusculoSkeletal Radiology Musculoskeletal Ultrasound Technical Guidelines III. Wrist Ian Beggs, UK Stefano Bianchi, Switzerland Angel Bueno, Spain Michel Cohen, France Michel Court-Payen, Denmark Andrew Grainger, UK Franz Kainberger, Austria Andrea Klauser, Austria Carlo Martinoli, Italy Eugene McNally, UK Philip J. O’Connor, UK Philippe Peetrons, Belgium Monique Reijnierse, The Netherlands Philipp Remplik, Germany Enzo Silvestri, Italy Wrist Note The standard US examination of the wrist begins with evaluation of its dorsal aspect, followed by the palmar one. Depending on the specific clinical presentation, US images can be obtained in different position of the wrist (flexion and extension, radial and ulnar deviation, pronation and supination), with the patient seated in front of the examiner. 1 DORSAL WRIST: compartments of extensor tendons Place the transducer on a transverse plane over the dorsal aspect of the wrist to allow proper identification of the extensor tendons. In general, one should first recognize a given tendon and then follow it on short-axis planes down to the distal insertion. Long- axis US images of the extensor tendons are less useful: they may help to evaluate the integrity of tendons and assess their dynamic motion in detail. Dynamic scanning of the extensor tendons can be performed by placing the hand on a gel tube with the fingers hanging outside its edge to allow easy fingers movements. Legend: APL, abductor pollicis longus; EPB, extensor pollicis brevis; ECRL, extensor carpi radialis longus; EPCB, extensor carpi radialis brevis; EPL, extensor pollicis longus; EIP, extensor indicis proprius; EDC, extensor digitorum longus; EDQ, extensor digiti quinti proprius; ECU, extensor carpi ulnaris 2 first compartment Keeping the patient’s wrist halfway between pronation and supination, place the probe over the lateral aspect of the radial styloid to examine the first compartment of the extensor tendons - abductor pollicis longus (ventral) and extensor pollicis brevis (dorsal). -

Versus Arthritis Carpal Tunnel Syndrome Information Booklet
Carpal tunnel syndrome Carpal tunnel syndrome information booklet Contents What is carpal tunnel syndrome? 4 What are the symptoms of carpal tunnel syndrome? 5 What causes carpal tunnel syndrome? 7 How is carpal tunnel syndrome diagnosed? 8 What treatments are there for carpal tunnel syndrome? 10 Self-help and daily living 15 Research and new developments 15 Where can I find out more? 16 Talk to us 17 We’re the 10 million people living with arthritis. We’re the carers, researchers, health professionals, friends and parents all united in our ambition to ensure that one day, no one will have to live with the pain, fatigue and isolation that arthritis causes. We understand that every day is different. We know that what works for one person may not help someone else. Our information is a collaboration of experiences, research and facts. We aim to give you everything you need to know about your condition, the treatments available and the many options you can try, so you can make the best and most informed choices for your lifestyle. We’re always happy to hear from you whether it’s with feedback on our information, to share your story, or just to find out more about the work of Versus Arthritis. Contact us at [email protected] Registered office: Versus Arthritis, Copeman House, St Mary’s Gate, Chesterfield S41 7TD Registered Charity England and Wales No. 207711, Scotland No. SC041156. Page 2 of 20 Page 3 of 20 Carpal tunnel syndrome information booklet What is carpal tunnel syndrome? What are the symptoms of carpal Carpal tunnel syndrome is a condition that happens when the tunnel syndrome? median nerve is compressed or squeezed as it passes through Carpal tunnel syndrome causes a tingling feeling or pins and needles, the wrist (see Figure 1). -

Carpal Tunnel Release: Avoiding Complications with Layer Shield Matrix
Ju ry [ rnal e ul rg d u e S C f h o i l r u a Journal of Surgery r n g r i u e o ] J ISSN: 1584-9341 [Jurnalul de Chirurgie] Research Article Open Access Carpal Tunnel Release: Avoiding Complications with Layer Shield Matrix Arrotegui JI* Departamento de Neurocirugía, Hospital General Universitario de Valencia, Spain Abstract Study design To evaluate the role played by the Layershield matrix (L.S.M.) in avoiding scar tissue and adhesion of the median nerve after decompression in carpal tunnel syndrome. Objective Prospective randomized trial to examine this technique. The idea was to investigate the potential benefits when dealing with complications (adherence of the flexor tendons and severing or scarring of the median nerve using the two- inch matrix as an adhesion barrier following mini-open carpal tunnel release). Summary of background The study cohort (L.S.M group) consisted of consecutive patients (200 patients) treated with L.S.M. Patients in the standard procedure group (200 patients in all) underwent operations using the same technique in carpal tunnel surgery in both groups, completing follow-up evaluations at no less than 3 to 6 months post-operation. The male to female ratio was 1:6. In twenty patients, there was bilateral involvement. Method All operations were conducted by the author at the Hospital General Universitario de Valencia, and the Clínica La Salud, Valencia, Spain, between 2012 and 2013. All patients complained of numbness and/or sensory disturbance or weakness in the median nerve distribution of the hand. Tinel and Phalen sign tests were positive in about two-thirds of patients. -

Wrist Problem Or Neck Problem? 8:00Am - 7:00Pm Wednesday - Carpal Tunnel Syndrome Is One of the and Arm Pain Or Tingling
ACTIVE P.T. SOLUTION S ...BECAUSE LIFE SHOULD BE ACTIVE APTS Monthly Office Hours: VOLUME VI, ISSUE IX SEPTEMBER 2016 Monday - 8:00am - 5:30pm Tuesday - Wrist Problem or Neck Problem? 8:00am - 7:00pm Wednesday - Carpal tunnel syndrome is one of the and arm pain or tingling. There are When the shoulders round and the most common nerve entrapments of some that get temporary relief but the head shifts forward, the muscles in 8:00am - 5:30pm the upper extremity. It occurs when problem recurs frequently with symp- the neck and shoulders compress Thursday - the median nerve is compressed in toms of higher intensity. Other pa- the nerves that course from the the wrist. However, it is not uncom- tients develop symptoms similar to neck to the hand. The hand re- 8:00am - 5:30pm mon for compression of the median carpal tunnel syndrome following neck quires a stable base at the neck and nerve to occur in several different injury. They may not have had a wrist shoulders in order to function Friday - sites in the forearm. Over the course injury but still experience pain in the properly. Weakness caused by 8:00am - 4:00pm of time, the general population has hand. inactivity and poor posture ulti- come to accept that hand and wrist mately causes the hand and fore- Location: pain, numbness, or tingling adds up to Since the body is a complex network arm muscles to be overworked. carpal tunnel syndrome. In fact, hun- of joints, nerves, ligaments, muscle, This results in “double crush syn- 91 Columbus Street dreds of people each year have wrist and fascia, it is possible that a symp- drome” or nerve compression in Auburn, NY 13021 decompression surgery in hopes of tom from one area of the body may be the neck and shoulders proximally relieving these symptoms. -

Carpal Tunnel Release Surgery
FACT SHEET FOR PATIENTS AND FAMILIES Carpal Tunnel Release Surgery What is carpal tunnel release surgery? Carpal tunnel release is surgery to treat carpal tunnel syndrome. Carpal tunnel syndrome is pain, weakness, tingling, and numbing in the thumb and fingers. It’s caused by pressure on the median nerve in the wrist. In carpal tunnel release surgery, the surgeon cuts the transverse carpal ligament, a band of tissue on the Transverse carpal palm side of the carpal tunnel. This takes pressure off ligament the median nerve and relieves symptoms. You will Released still be able to use your wrist and hand, and in time, Median carpal nerve they should work as if you never had a problem. tunnel Why do I need it? Before carpal tunnel release surgery, the transverse Most cases of carpal tunnel syndrome are treated carpal ligament is putting pressure on the median nerve and causing pain and weakness. During without surgery. Your doctor may recommend surgery, the ligament is cut to take pressure off surgery if: the nerve. • You have tried nonsurgical treatments for several weeks or months and your symptoms have not improved. Talking with your doctor • Your symptoms are severe and interfere with The table below lists the most common potential your daily activities. benefits, risks, and alternatives for carpal tunnel release • Your median nerve is damaged. surgery. There may be other benefits or risks in your • Other tissue, such as a tumor, is putting pressure unique medical situation. Talking with your doctor is on the median nerve. the most important part of learning about these risks and benefits. -

036040063E.Pdf
Original Study Workers’ Compensation, Return to Work, The Role of and Patient Satisfaction After Carpal Aspirin Tunnel Decompression Keith R Ramsey A. Ellis, MD, Christine B. Novak, PT, MS, Susan E. Mackinnon, MD, and Christine J. Cheng, MD injuries have shown that psychosocial and job factors rather than physical factors are better predictors of return Abstract to work.18,19 Similar results have been reported in studies In the study reported here, we assessed satisfaction and of workers with CTS.11,12,20 Although WC and non-WC return to work in workers’ compensation (WC) patients patients have reported similar levels of satisfaction with after carpal tunnel decompression. Eighty of the 362 surgery, WC patients take longer to return to work and are patients who underwent surgery met the study criteria; less likely to return to work.5,21 42 of the 80 were found for follow-up; 40 of the 42 In the study reported here, we evaluated return to work and participated in the telephone questionnaire; 15 (38%) of patient satisfaction with surgical and job-related factors after the 40 received WC; and 39 (98%) of the 40 returned to primary CTD in both WC patients and non-WC patients. work. Mean age of the 40 respondents was 47 years, and mean follow-up was 29 months. WC involvement was not related to return to work and did not affect satisfaction MATERIALS AND METHODS with overall outcome but was related to dissatisfaction Patient Selection with job factors and timing of return to work. After obtaining institutional Human Studies Committee approval, we retrospectively reviewed the patient records of a surgeon (Dr. -

Carpal Tunnel Syndrome
Oxford University Hospitals NHS Trust Hand & Plastics Physiotherapy Department Carpal Tunnel Syndrome Information for patients page 2 What is the Carpal Tunnel? The carpal tunnel is made up of the bones in your wrist and a ligament which runs across the base of your palm. Several tendons and your ‘median nerve’ run through the tunnel to supply movement and sensation to your fingers. What causes Carpal Tunnel Syndrome? Symptoms occur when the nerve becomes ‘pinched’ by pressure within the tunnel. The reason is usually unknown, but possible causes can include: swelling of the lining of the tendons, joint dislocation, fractures or arthritis. Fluid retention during pregnancy can also sometimes cause swelling in the tunnel. Symptoms are made worse by keeping the wrist bent for long periods of time. What are the symptoms? Symptoms include numbness, tingling and/or pain in the arm, hand and/or fingers of the affected side. The symptoms are more often felt during the night, but may be noticed during the day when the wrist is bent for long periods of time. You may have noticed a weaker grip, or clumsiness when using your hand. In severe cases sensation may be permanently lost, and the muscles at the base of the thumb may reduce in size. Diagnosis A clinician may do a test such as tapping along the line of the nerve or bending your wrist to see if your symptoms are brought on. You may also be sent for nerve conduction studies to give an accurate measure of the degree of pressure that is affecting the nerve. -

Information for Patients About Hand & Elbow Surgery
Information for Patients about Hand & Elbow Surgery Clinical Professor Allan Wang FRACS PhD FAOrthA Shoulder and Upper Limb Surgeon www.allanwangorthopaedics.com.au MURDOCH SUBIACO Murdoch Orthopaedic Clinic St John of God Subiaco Clinic St John of God Murdoch Clinic Suite 302, 25 McCourt St Suite 10, 100 Murdoch Drive Subiaco WA 6008 Murdoch WA 6150 Telephone: 08 6332 6390 Page | 2 Page | 3 Information for Patients about Hand and Elbow Surgery Introduction We have put this information booklet together to educate our patients about their Hand and Elbow condition, treatment options and post-surgical care. Please keep this booklet for future reference. It is not a detailed source of information and you may also wish to refer to our website www.allanwangorthopaedics.com.au for animated videos of surgical procedures. If you require further information or have concerns regarding your treatment please contact the office to discuss with Dr Wang or his staff. Contents Pages 1. Carpal Tunnel Syndrome 4 2. Cubital Tunnel Syndrome 6 3. Trigger Finger 7 4. De Quervain’s Tenodonitis 8 5. Ganglion Cysts 9 6. Arthritis at the Base of the Thumb 10 7. Wrist Arthroscopy 11 8. Dupuytren’s Disease 12 9. Lateral Epicondylitis 13 10. Elbow Arthroscopy 14 11. Post-Operative Instructions Hand & Elbow Surgery 15 Page | 4 Carpal Tunnel Syndrome What is it? Figure 1 Carpal tunnel syndroe is a condition aused by copression o te median nerve at te level o te wrist oint Here te edian nerve passes into te arpal tunnel along wit leor tendons and te tendon lining called tenosynoviu Carpal tunnel syndroe ocurs wen pressure builds up in te tunnel and tis an be due to swelling o te tenosynoviu ratures artritis luid retention during pregnany and certain conditions suc as diabetes and tyroid disease Symptoms en te pressure on te edian nerve becoes severe, you ay notice wrist pain tingling and nubness and lusiness in and oveents. -

Carpal Tunnel Syndrome and Ulnar Neuropathy at Elbow
PERIPHERAL NERVE ENTRAPMENTS CTS Ulnar Neuropathy at elbow CARPAL TUNNEL SYNDROME • Compression of the median nerve in a fibro- osseus canal on the palmar surface of the wrist: the carpal tunnel. • Most common entrapment neuropathy • 2-3% of the population. • Variations in prevalence data is likely due to variations in occupational exposure. • 90% have a good outcome and are able to work again and 10 % are permanently disable. Carpal Tunnel Anatomy • Fibro-osseus tunnel bounded by the carpal bones, the interosseus ligaments and the transverse carpal ligament (the flexor retinaculum). • Content: Median nerve, tendons of flexor digitorum superficialis (FDS), tendons of flexor digitorum profundus (FDP), and tendon of flexor pollicis longus (FPL). The Flexor Retinaculum • Extends 1 cm or more proximal to the most distal wrist crease distally at least 3 to 4 cm into the palm. • Constituted by the fusion of the TCL and deeper transverse fibers of the palmar aponeurosis. Palmar Cutaneous Branch • Originates from the median nerve before it enters to the carpal tunnel. • Exits the median nerve along its anterolateral quadrant about 3 to 4 cm above the distal wrist crease. • Passes superficial to the TCL. • Supplies sensation to the proximal surface of the thenar eminence. Recurrent Motor Branch • Leaves the radial side of the median nerve distal to the flexor retinaculum. • Curves back around to enter the thenar muscle mass. • Multiple anatomical variations. • 31% of cases: leaves the ulnar side of the median nerve beneath the TCL • 20 % of cases: transligamentous course. Surgical Anatomy Kaplan’s Cardinal Line: • Apex of the First web space (between the thumb and index finger) • Parallel with the proximal palmar crease.