Cystophora Cristata) Pups During Extreme Lactation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marine Mammals of Hudson Strait the Following Marine Mammals Are Common to Hudson Strait, However, Other Species May Also Be Seen
Marine Mammals of Hudson Strait The following marine mammals are common to Hudson Strait, however, other species may also be seen. It’s possible for marine mammals to venture outside of their common habitats and may be seen elsewhere. Bowhead Whale Length: 13-19 m Appearance: Stocky, with large head. Blue-black body with white markings on the chin, belly and just forward of the tail. No dorsal fin or ridge. Two blow holes, no teeth, has baleen. Behaviour: Blow is V-shaped and bushy, reaching 6 m in height. Often alone but sometimes in groups of 2-10. Habitat: Leads and cracks in pack ice during winter and in open water during summer. Status: Special concern Beluga Whale Length: 4-5 m Appearance: Adults are almost entirely white with a tough dorsal ridge and no dorsal fin. Young are grey. Behaviour: Blow is low and hardly visible. Not much of the body is visible out of the water. Found in small groups, but sometimes hundreds to thousands during annual migrations. Habitat: Found in open water year-round. Prefer shallow coastal water during summer and water near pack ice in winter. Killer Whale Status: Endangered Length: 8-9 m Appearance: Black body with white throat, belly and underside and white spot behind eye. Triangular dorsal fin in the middle of the back. Male dorsal fin can be up to 2 m in high. Behaviour: Blow is tall and column shaped; approximately 4 m in height. Narwhal Typically form groups of 2-25. Length: 4-5 m Habitat: Coastal water and open seas, often in water less than 200 m depth. -

The Grey Seal
Factsheet: The grey seal Happy Horsey Seal by Mary Groombridge Where can grey seals be found? The grey seal is the larger and more common of the two British seal species, the other being the common seal (aka harbour seal). There are 3 distinct populations of grey seals in the world, but it is the eastern Atlantic population that is mainly found in the UK. One hundred years ago there were only around 500 grey seals in this country. Now however, half of the world’s population, approximately 80,000 individuals, are found on and around British coasts. They are usually found mainly around exposed rocky northern and western coasts, however the wide, sandy beaches in Norfolk provide an important breeding area for them. What do grey seals look like? Grey seals are classed as ‘true seals’, meaning that they have no external ears and have shorter front flippers. Unlike ‘eared seals’ such as sea lions, grey seals are less mobile on land and tend to move along the ground on their belly. The grey seal can be distinguished from the common seal by its long, straight ‘Roman’ nose and wide nostrils earning its scientific name Halichoerus grypus, meaning "hooked-nosed sea pig". Common seals have smaller, rounder heads with shorter noses. Adult grey seals can grow up to 2.5 metres long; males are much larger than females, averaging 233kg in weight, while females average around 155kg. Males are generally darker in colour and often scarred from territorial battles with other males. For this reason males rarely live longer than 25 years, while females can live for up to 35 years. -

December 20, 2007
BEFORE THE SECRETARY OF COMMERCE PETITION TO LIST THE RIBBON SEAL (HISTRIOPHOCA FASCIATA) AS A THREATENED OR ENDANGERED SPECIES UNDER THE ENDANGERED SPECIES ACT © G. CARLETON RAY CENTER FOR BIOLOGICAL DIVERSITY DECEMBER 20, 2007 Notice of Petition____________________________________________________ Carlos M. Gutierrez Secretary of Commerce U.S. Department of Commerce 1401 Constitution Avenue, N.W., Room 5516 Washington, D.C. 20230 Dr. William Hogarth Assistant Administrator for Fisheries National Oceanographic and Atmospheric Administration 1315 East-West Highway Silver Springs, MD 20910 PETITIONER The Center for Biological Diversity 1095 Market Street, Suite 511 San Francisco, CA 94103 ph: (415) 436-9682 ext 301 fax: (415) 436-9683 __________________________ Date: this 20th day of December, 2007 Shaye Wolf, Ph.D. Martha Palomino Tovar, Ph.D. Candidate Brendan Cummings Center for Biological Diversity Pursuant to Section 4(b) of the Endangered Species Act (“ESA”), 16 U.S.C. §1533(b), Section 553(3) of the Administrative Procedures Act, 5 U.S.C. § 553(e), and 50 C.F.R. §424.14(a), the Center for Biological Diversity (“Petitioner”) hereby petitions the Secretary of Commerce, through the National Marine Fisheries Service (“NMFS”), to list the ribbon seal (Histriophoca fasciata) as a threatened or endangered species and to designate critical habitat to ensure its survival and recovery. The Center for Biological Diversity (“Center”) is a non-profit, public interest environmental organization dedicated to the protection of native species and their habitats through science, policy, and environmental law. The Center has over 40,000 members in Alaska and throughout the United States. The Center and its members are concerned with the conservation of endangered species, including the ribbon seal, and the effective implementation of the ESA. -

Behaviors of Grey Seals (<I>Halichoerus Grypus</I>)
Tourism in Marine Environments, Vol. 15, No. 3–4, pp. 159-171 1544-273X/20 $60.00 + .00 Printed in the USA. All rights reserved. DOI: https://doi.org/10.3727/154427320X15945013137030 Copyright © 2020 Cognizant, LLC. E-ISSN 2169-0197 www.cognizantcommunication.com BEHAVIORS OF GREY SEALS (HALICHOERUS GRYPUS) ADDRESSED TOWARDS HUMAN SWIMMERS DURING EXPERIMENTAL OPEN WATER ENCOUNTERS OFF HELIGOLAND (GERMAN BIGHT, NORTH SEA) MICHAEL SCHEER Independent Scholar, Bremen, Germany Risks arising for humans during swim encounters with seals are poorly understood. This study was initiated to examine behaviors of unhabituated grey seals addressed towards humans during experi- mental, noncommercial seal-swim activities off Heligoland. In total, 26 in-water encounters were conducted. Behavioral classes and the number of seals simultaneously approaching swimmers were time sampled. A set of risky and nonrisky interactive behaviors was continuously sampled. Seals spent approximately the same amount of time interacting with swimmers (53%) as they did ignoring them (47%). Seals displayed higher rates of nonrisky behaviors than risky ones, but risky behaviors occurred during 73% of all seal-swims. Seals remained ≤20 m near swimmers for 51% and ≤1 m for 13% of the time. A mean number of 0.65 and 0.18 seals approached swimmers per minute within a range of ≤20 m and ≤1 m, respectively. Behavioral classes, interactive behaviors, and the number of seals approaching ≤20 m did not vary significantly throughout seal-swims but the number of seals approaching ≤1 m moderately decreased. Due to high rates of risky behaviors, it is recommended to promote public awareness on site and to regulate seal-swims before commercial operations emerge. -

The Conflict Between Grey Seals (Halichoerus Grypus) and the Baltic Coastal Fisheries - New Methods for the Assessment and Reduction of Catch Losses and Gear Damage
The conflict between grey seals (Halichoerus grypus) and the Baltic coastal fisheries - new methods for the assessment and reduction of catch losses and gear damage Cover picture: Grey seal (Halichoerus grypus) taking fish from a herring net. A four camera system with CamDisc Recorder and HelTel Player software was used. Photo S. Königson. September 2004. ISBN 91-85497-30-4 ISSN 0345-7524 Printed by LiU-Tryck, Linköping 2006 ABSTRACT There is a problematic interaction going on between grey seals and the small scale coastal fisheries in the Baltic. A large number of seals are by-caught and drowned each year, and the viability of the fishery is threatened by catch losses caused by the seals. Traditional mitigation methods are not sufficient, or have in some cases not been properly evaluated. Available methods of quantifying and analysing the catch losses are also insufficient. This thesis consists of three parts, each studying a different angle of this conflict. In the first part, new models for estimating catch losses are presented. In addition to the commonly used method of counting the number of damaged fish in the nets, the new models also allow for an estimation of the hidden losses. Hidden losses may be fish that are completely removed from nets without leaving any traces, fish that escape through holes in the net torn by the seals, or even fish that are scared away from the fishing gear. Such losses were found to be significant, and hence it is now clear that the traditional models seriously underestimate the total losses. The new models also allow for a deeper analysis of the interaction process. -

Full Text in Pdf Format
Vol. 16: 149–163, 2012 ENDANGERED SPECIES RESEARCH Published online February 29 doi: 10.3354/esr00392 Endang Species Res Age estimation, growth and age-related mortality of Mediterranean monk seals Monachus monachus Sinéad Murphy1,*, Trevor R. Spradlin1,2, Beth Mackey1, Jill McVee3, Evgenia Androukaki4, Eleni Tounta4, Alexandros A. Karamanlidis4, Panagiotis Dendrinos4, Emily Joseph4, Christina Lockyer5, Jason Matthiopoulos1 1Sea Mammal Research Unit, Scottish Oceans Institute, University of St. Andrews, St. Andrews, Fife KY16 8LB, UK 2NOAA Fisheries Service/Office of Protected Resources, Marine Mammal Health and Stranding Response Program, 1315 East-West Highway, Silver Spring, Maryland 20910, USA 3Histology Department, Bute Medical School, University of St. Andrews, St. Andrews, Fife KY16 9TS, UK 4MOm/Hellenic Society for the Study and Protection of the Monk Seal, 18 Solomou Street, 106 82 Athens, Greece 5Age Dynamics, Huldbergs Allé 42, Kongens Lyngby, 2800, Denmark ABSTRACT: Mediterranean monk seals Monachus monachus are classified as Critically Endan- gered on the IUCN Red List, with <600 individuals split into 3 isolated sub-populations, the largest in the eastern Mediterranean Sea. Canine teeth collected during the last 2 decades from 45 dead monk seals inhabiting Greek waters were processed for age estimation. Ages were best estimated by counting growth layer groups (GLGs) in the cementum adjacent to the root tip using un - processed longitudinal or transverse sections (360 µm thickness) observed under polarized light. Decalcified and stained thin sections (8 to 23 µm) of both cementum and dentine were inferior to unprocessed sections. From analysing patterns of deposition in the cementum of known age- maturity class individuals, one GLG was found to be deposited annually in M. -
Global Patterns in Marine Mammal Distributions
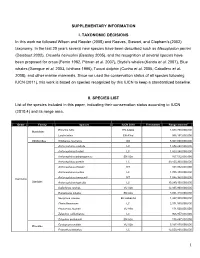
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

Novel Terrestrial Haul-Out Behaviour by Ringed Seals
POLAR RESEARCH, 2017 VOL. 36, 1374124 https://doi.org/10.1080/17518369.2017.1374124 RESEARCH ARTCLE Novel terrestrial haul-out behaviour by ringed seals (Pusa hispida)in Svalbard, in association with harbour seals (Phoca vitulina) Christian Lydersena, Jade Vaquie-Garciaa, Espen Lydersenb, Guttorm N. Christensenc & Kit M. Kovacsa aNorwegian Polar Institute, Tromsø, Norway; bUniversity College of Southeast Norway, Campus Bø, Norway; cAkvaplan-Niva, Tromsø, Norway ABSTRACT KEYWORDS Ringed seals (Pusa hispida) are the most ice-associated of all Arctic pinnipeds. In the Svalbard Arctic; behavioural plasticity; area, this species has always given birth, moulted and rested on sea ice. In addition, much of climate change; glacier their food has been comprised of ice-associated prey. Recently, ringed seals have been fronts; lagoons; sea ice reported to be using terrestrial substrates as a haul-out platform in some fjords on the west coast of Spitsbergen. In many cases the seals involved are harbour seals (Phoca vitulina), which are extending their distribution into new areas within the Svalbard Archipelago and which are being misclassified as ringed seals. However, this study reports that terrestrial haul- out by ringed seals is also now taking place on rocks exposed at low tide as well as on the coastline. Recent intrusions of warm Atlantic Water (with associated prey) have extended deep into the fjords of western Spitsbergen, resulting in deteriorated ice conditions for ringed seals and expanded habitat for harbour seals. Over the last decade, ringed seals have become more and more confined in coastal areas to narrow bands in front of tidal glacier fronts where Arctic conditions still prevail. -

UPDATE MARINE MAMMALS Circumpolar Marine Mammal Expert Group, CBMP-Marine
State of the Arctic Marine Biodiversity Report UPDATE MARINE MAMMALS Circumpolar Marine Mammal Expert Group, CBMP-Marine 2021 Polar bear with northern lights, Canada. Photo credit: Ondrej Prosicky/Shutterstock.com In 2017, the State of the Arctic Marine Biodiversity Report (SAMBR) synthesized data about biodiversity in Arctic marine ecosystems around the circumpolar Arctic. SAMBR highlighted observed changes The Circumpolar Biodiversity and relevant monitoring gaps. This document provides an update on Monitoring Program (CBMP) is the status of marine mammals in the circumpolar Arctic from 2015– an adaptive monitoring 2020 (scientific references for factual information and a more detailed program based on an version of the text herein can be found in Kovacs et al. 2021). international network of scientists, government Arctic endemic marine mammals are one focal group of the Circumpolar agencies, Indigenous Biodiversity Monitoring Program’s (CBMP) Arctic Marine Biodiversity organizations and conservation Monitoring Plan (CBMP-Marine Plan), along with sea ice biota, plankton, groups working together to benthos, marine fishes and seabirds. Networks of experts have harmonize and integrate efforts identified key elements, called Focal Ecosystem Components (FECs), to monitor the Arctic’s living within the Arctic marine ecosystem. Changes in the status of FECs resources. The CBMP organizes indicate changes in the broader marine environment. its efforts around the major This update was prepared by the Marine Mammals Expert Network, ecosystems of the Arctic: which works to coordinate monitoring and report scientific findings marine, freshwater, terrestrial regarding trends and their drivers in marine mammal populations and coastal. around the Arctic. In 2011, the CBMP Marine Expert Monitoring Group BACKGROUND published a circumpolar monitoring plan that describes Arctic endemic marine mammals live in close association with sea how Arctic states will compile, ice. -

Electrophoretic Variation in Large Mammals. III. the Ringed Seal, Pusa-Hispida, the Harp Seal, Pagophilus-Groenlandicus, and the Hooded Seal, Cystophora-Cristata
University of Montana ScholarWorks at University of Montana Biological Sciences Faculty Publications Biological Sciences 1982 Electrophoretic Variation in Large Mammals. III. The Ringed Seal, Pusa-Hispida, the Harp Seal, Pagophilus-Groenlandicus, and the Hooded Seal, Cystophora-Cristata V. Simonsen Fred W. Allendorf University of Montana - Missoula, [email protected] W. F. Eanes F. O. Kapel Follow this and additional works at: https://scholarworks.umt.edu/biosci_pubs Part of the Biology Commons Let us know how access to this document benefits ou.y Recommended Citation Simonsen, V.; Allendorf, Fred W.; Eanes, W. F.; and Kapel, F. O., "Electrophoretic Variation in Large Mammals. III. The Ringed Seal, Pusa-Hispida, the Harp Seal, Pagophilus-Groenlandicus, and the Hooded Seal, Cystophora-Cristata" (1982). Biological Sciences Faculty Publications. 63. https://scholarworks.umt.edu/biosci_pubs/63 This Article is brought to you for free and open access by the Biological Sciences at ScholarWorks at University of Montana. It has been accepted for inclusion in Biological Sciences Faculty Publications by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. Hereditas 97: 87-90 (1982) Electrophoretic variation in large mammals 111. The ringed seal, Pusa hispida, the harp seal, Pagophilus groenlandicus, and the hooded seal, Cystophora cristata. V. SIMONSEN', F. W. ALLENDORF, W. F. EANES3 and F. 0. KAPEL4 ' Institute of Ecology and Genetics, University of Aarhus, Denmark Department of Zoology, University of Montana, USA ' Department of Ecology and Evolution, State University of New York, Stony Brook, USA Greenland Fisheries Investigations, Charlottenlund, Denmark SIMONSEN, V., ALLENDORF, F. W.,EANES, W. -

Pacific Walrus (Odobenus Rosmaurs Divergens) As a Threatened Or Endangered Species Under the Endangered Species Act
BEFORE THE SECRETARY OF INTERIOR PETITION TO LIST THE PACIFIC WALRUS (ODOBENUS ROSMAURS DIVERGENS) AS A THREATENED OR ENDANGERED SPECIES UNDER THE ENDANGERED SPECIES ACT © BILL HICKEY, USFWS CENTER FOR BIOLOGICAL DIVERSITY FEBRUARY 7, 2008 Notice of Petition____________________________________________________ Dirk Kempthorne, Secretary Department of the Interior 1849 C Street, N.W. Washington. D.C. 20240 Tom Melius, Regional Director U.S. Fish and Wildlife Service Alaska Regional Office 1011 East Tudor Road Anchorage, Alaska 99503 PETITIONER The Center for Biological Diversity 1095 Market Street, Suite 511 San Francisco, CA 94103 ph: (415) 436-9682 ext 301 fax: (415) 436-9683 __________________________ Date: this 7th day of February, 2008 Shaye Wolf, Ph.D. Kassie Siegel Brendan Cummings Center for Biological Diversity Pursuant to Section 4(b) of the Endangered Species Act (“ESA”), 16 U.S.C. §1533(b), Section 553(3) of the Administrative Procedures Act, 5 U.S.C. § 553(e), and 50 C.F.R. § 424.14(a), the Center for Biological Diversity hereby petitions the Secretary of the Interior, through the United States Fish and Wildlife Service (“USFWS”), to list the Pacific walrus (Odobenus rosmarus divergens) as a threatened or endangered species and to designate critical habitat to ensure its survival and recovery. The Center for Biological Diversity works through science, law, and policy to secure a future for all species, great or small, hovering on the brink of extinction. The Center has over 40,000 members throughout Alaska and the United States. The Center and its members are concerned with the conservation of endangered species, including the Pacific walrus, and the effective implementation of the ESA. -

Current Status of Pinniped Conservation Research in KOREA
Current status of Pinniped conservation research in KOREA Seong Oh Im1), Hye-min Park1), InSeo Hwang1), Sang Heon Lee2), Younggeun Oh2) , Hyun-Woo Kim3), Eun-Bi Kim3) 1)Korea Marine Environment Management Corporation, 2)Pusan National University, 3)Pukyong National University * Correspondence: Korea Marine Environment Management Corporation / * E-mail: [email protected] 1. Introduction Six pinniped species are currently designated as Marine protected species (MPS) in KOREA. Their main breeding grounds are known to be China and Russia, and the waters along the Korean peninsula are considered as the marginal region for those species with extremely low individuals. Among them, Researches have been limited to the spotted seals (Phoca largha), the most abundant pinniped species in the Yellow Sea, Korea. Most of their studies have been mainly dependent on the traditional monitoring methods from visual surveys to count individuals of air-breathing vertebrates, which required a high-degree of labors and costs. As one of promising novel alternative tools for the traditional surveys, molecular biological analyses using environmental DNA (eDNA) has been paid attention for marine ecological studies due to its low cost and labors and high sensitivity in taxon recovery. We here introduce current status of research for pinniped conservation in Republic of Korea using both traditional and novel molecular tools. In addition, satellite monitoring results and environmental conditions around breeding grounds are compared and analyzed. Current status of Pinniped conservation research in KOREA (A) Donghae (A) Donghae 2. Materials & Methods (B) Ulleung ① Visual surveys 3 times of field survey (May, (C) Dokdo August, October, 2020) in the East Sea of Korea (B) Ulleung (Donghae, Ulleung Island, and Dokdo).