Hydrothermal Faunal Assemblages and Habitat Characterisation at The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Case Reports for Species & Habitats on the Initial Draft
OSPAR Commission, 2008: Case Reports for the OSPAR List of Threatened and/or Declining Species and Habitats _______________________________________________________________________________________ Nomination earths crust through channels formed in cooling Oceanic ridges with hydrothermal lava flows. The tall chimneys formed around the vents/fields vents and the surrounding sediments are almost EUNIS code: A6.94 pure metallic sulphides and are a unique geological feature of hydrothermal vents National Marine Habitat Classification for UK (Tunnicliffe et al., 1998). The associated animal & Ireland code: Not defined communities are particularly unusual as the species derive energy under conditions where Definition for habitat mapping photosynthesis is not possible, tolerate great Hydrothermal vents occur along spreading ridges extremes and variability in the temperature and (such as the mid-Atlantic ridge), subduction the chemical composition of the surrounding zones, fracture zones and back-arc basins (Gage water, and cope with potentially toxic & Tyler, 1991), and are caused by seawater concentrations of various heavy metals. penetrating the upper levels of the Earth’s crust through channels formed in cooling lava flows, Application of the Texel-Faial criteria reacting chemically with hot basalt in the Earth’s Hydrothermal vents/fields were nominated in a crust and then rising back to the sea-bed to vent joint submission by three Contracting Parties as superheated water containing compounds such citing regional importance, decline, rarity, and as sulphides, metals, CO2 and methane sensitivity, with information also provided on et al (Tunnicliffe , 1998 in Gubbay, 2002). The threat. The nomination was for Region V. water may trickle out from cracks and crevices on the seabed as hot springs (5-250°C), or as very Regional importance concentrated jets of superheated water (270- 380°C). -

Tom 59 2010 Numer 1–2 (286–287) Strony 39–59
Tom 59 2010 Numer 1–2 (286–287) Strony 39–59 Piotr Jóźwiak, tomasz rewicz, krzysztof Pabis Katedra Zoologii Bezkręgowców i Hydrobiologii Uniwersytet Łódzki Banacha 12/16, 90-237 Łódź E-mail: [email protected] [email protected] [email protected] INSPIRACJE I OSOBLIWOŚCI NAUKOWEGO NAZEWNICTWA ZOOLOGICZNEGO WSTĘP Świat przyrody z jego bogactwem form szczególne organizmy jak ich: krwistość lub oraz mnogością zachodzących w nim zjawisk bezkrwistość, pokrycie ciała (owłosienie lub od najdawniejszych czasów był obiektem pióra), liczbę nóg (dwu- lub czteronożność), zainteresowania ze strony człowieka. Już w czy obecność skorupy. Co więcej Arystote- starożytności podejmowane były próby ba- les w swoich badaniach nie ograniczał się dania różnorodności tego świata. Pojawiły jedynie do morfologii organizmów, lecz brał się pierwsze opisy roślin i zwierząt, a po- pod uwagę także ich inne właściwości takie szczególnym gatunkom zaczęto nadawać na- jak zachowanie: „Zwierzęta mogą być cha- zwy. Te gatunki, które wykazywały wzajemne rakteryzowane przez swój tryb życia, swoje podobieństwa, grupowano tworząc zalążki czynności, zwyczaje i części ciała”. Z systemu systematyki. Pierwotnie nazewnictwo i kla- klasyfikacji Arystotelesa do czasów współcze- syfikacja organizmów uwarunkowana była snych zachowały się takie nazwy jak Coleop- ich użytecznością dla człowieka — tym, czy tera (chrząszcze) oraz Diptera (muchówki), a są one jadalne, czy mają właściwości leczni- także stosowany jeszcze do niedawna podział cze, czy też stanowią zagrożenie jako trujące owadów na Pterygota (owady uskrzydlone) i bądź jadowite. Przykładem mogą być też nie- Apterygota (owady bezskrzydłe) (mayr 1974, które współczesne plemiona z Ameryki Połu- Huxley 2009). dniowej. Indianie ci potrafią rozpoznawać i Na przestrzeni wieków, wraz z rozwojem nazwać szereg wykorzystywanych przez sie- metod badawczych, klasyfikacja i nazewnic- bie gąsienic różnych gatunków motyli. -

SUPPLEMENTARY INFORMATION Ecology and Biogeography Of
Title Ecology and biogeography of megafauna and macrofauna at the first known deep-sea hydrothermal vents on the ultraslow- spreading Southwest Indian Ridge Authors Copley, JT; Marsh, L; Glover, AG; Hühnerbach, V; Nye, VE; Reid, WDK; Sweeting, CJ; Wigham, BD; Wiklund, H Description 0000-0002-9489-074X Date Submitted 2017-05-02 SUPPLEMENTARY INFORMATION Ecology and biogeography of megafauna and macrofauna at the first known deep-sea hydrothermal vents on the ultraslow-spreading Southwest Indian Ridge Copley JT 1,* , Marsh L 1, Glover AG 2, Hühnerbach V 3, Nye VE 1, Reid WDK 4, Sweeting CJ 5, Wigham BD 5, Wiklund H 2 1Ocean & Earth Science, University of Southampton, Waterfront Campus, European Way, Southampton SO14 3ZH, UK 2Life Sciences Department, Natural History Museum, Cromwell Road, London SW7 5BD, UK 3formerly at National Oceanography Centre, European Way, Southampton SO14 3ZH, UK 4School of Biology, Newcastle University, Newcastle Upon Tyne NE1 7RU, UK 5Dove Marine Laboratory, School of Marine Science & Technology, Newcastle University, Cullercoats NE30 4PZ, UK *email [email protected] (corresponding author) SUPPLEMENTARY FIGURE: Images of faunal assemblages observed at Longqi vent field, Southwest Indian Ridge, during the first remotely operated vehicle (ROV) dives in November 2011: (a) active “black smoker” chimneys occupied by Rimicaris kairei ; (b) assemblage of Chrysomallon squamiferum , Hesiolyra cf. bergi , Kiwa n. sp. “SWIR”, Mirocaris fortunata in close proximity to vent fluid source; (c) abundant Chrysomallon squamiferum and Gigantopelta aegis , with Kiwa n. sp. “SWIR”, Bathymodiolus marisindicus , and Mirocaris fortunata on platform of “Tiamat” vent chimney (d) zonation of Chysomallon squamiferum , Gigantopelta aegis , Bathymodiolus marisindicus , and Neolepas sp. -

AMPHIPOD Newsletter 35 (2011)
Amphipod Newsletter 35 ! ! 2011 AMPHIPOD newsletter 35 (2011) Introduction of the Bibliography In remembrance Meetings & info new AN-team The list of new amphipod George Crawford XIV ICA 2010 who will take over the literature since Amphipod Stella Vassilenko XV ICA 2013 Amphipod Newsletter? Newsletter 34 (2010) TCS summer 2012 Page 2 Pages 3 - 43 Pages 44 - 45 Pages 46 - 47 INTRODUCTION The Amphipod Newsletter was started up by me in 1971, and in 40 years I have, often with the help of colleagues, produced 35 issues, at first mimeographed, the later ones on the Amphipod Website on the Internet. I have retired from my position as curator of zoology at Tromsø Museum in Tromsø, N. Norway, 4 years ago on reaching 70, and at the latest amphipod conference, in Sevilla in Spain in September 2010, I have asked for more regular assistance in the production of the newsletter, even though I am willing to continue for the time being to assist in the compiling of a bibliography of recent amphipod literature. At the meeting help was indeed offered, and from this issue, AN 35, the newsletter will be produced in cooperation with Adam Baldinger (Harvard), Miranda Lowe (Natural History Museum, London) and Anne Helene S. Tandberg (Norwegian Institute of New Amphipod Web-Site Marine Research, Tromsø, Norway). They will, maybe unnecessarily for most of you, introduce themselves elsewhere in this newsletter. A new amphipod web-site is Also the Amphipod website will move from Tromsø to Harvard eventually and under construction at as soon as some practical difficulties have been overcome. -

First Observations of the Behaviour of the Deep-Sea Amphipod Dulichiopsis Dianae Sp
First observations of the behaviour of the deep-sea amphipod Dulichiopsis dianae sp. nov. (Senticaudata, Dulichiidae) in the TAG hydrothermal vent field (Mid-Atlantic Ridge) Laure Corbari, Jean-Claude Sorbe To cite this version: Laure Corbari, Jean-Claude Sorbe. First observations of the behaviour of the deep-sea amphipod Duli- chiopsis dianae sp. nov. (Senticaudata, Dulichiidae) in the TAG hydrothermal vent field (Mid-Atlantic Ridge). Marine Biodiversity, Springer Verlag, 2017, 10.1007/s12526-017-0788-y. hal-01618749 HAL Id: hal-01618749 https://hal.sorbonne-universite.fr/hal-01618749 Submitted on 18 Oct 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. First observations of the behaviour of the deep-sea amphipod Dulichiopsis dianae sp. nov. (Senticaudata, Dulichiidae) in the TAG hydrothermal vent field (Mid-Atlantic Ridge) 1 2 Laure Corbari & Jean-Claude Sorbe Abstract A Btiny and mysterious creature swinging on a Keywords Amphipoda . Dulichiopsis . Behaviour . stem^: that was the first observation of the new species Hydrothermal vents . Deep sea Dulichiopsis dianae sp. nov. made during the remotely oper- ated vehicle (ROV) dive devoted to the exploration of the surroundings of the vent site TAG (BICOSE cruise, 3550– Introduction 3650 m). -

Supplementary Information
Title Ecology and biogeography of megafauna and macrofauna at the first known deep-sea hydrothermal vents on the ultraslow- spreading Southwest Indian Ridge Authors Copley, JT; Marsh, L; Glover, AG; Hühnerbach, V; Nye, VE; Reid, WDK; Sweeting, CJ; Wigham, BD; Wiklund, H Description 0000-0002-9489-074X Date Submitted 2017-05-02 SUPPLEMENTARY INFORMATION Ecology and biogeography of megafauna and macrofauna at the first known deep-sea hydrothermal vents on the ultraslow-spreading Southwest Indian Ridge Copley JT 1,* , Marsh L 1, Glover AG 2, Hühnerbach V 3, Nye VE 1, Reid WDK 4, Sweeting CJ 5, Wigham BD 5, Wiklund H 2 1Ocean & Earth Science, University of Southampton, Waterfront Campus, European Way, Southampton SO14 3ZH, UK 2Life Sciences Department, Natural History Museum, Cromwell Road, London SW7 5BD, UK 3formerly at National Oceanography Centre, European Way, Southampton SO14 3ZH, UK 4School of Biology, Newcastle University, Newcastle Upon Tyne NE1 7RU, UK 5Dove Marine Laboratory, School of Marine Science & Technology, Newcastle University, Cullercoats NE30 4PZ, UK *email [email protected] (corresponding author) SUPPLEMENTARY FIGURE: Images of faunal assemblages observed at Longqi vent field, Southwest Indian Ridge, during the first remotely operated vehicle (ROV) dives in November 2011: (a) active “black smoker” chimneys occupied by Rimicaris kairei ; (b) assemblage of Chrysomallon squamiferum , Hesiolyra cf. bergi , Kiwa n. sp. “SWIR”, Mirocaris fortunata in close proximity to vent fluid source; (c) abundant Chrysomallon squamiferum and Gigantopelta aegis , with Kiwa n. sp. “SWIR”, Bathymodiolus marisindicus , and Mirocaris fortunata on platform of “Tiamat” vent chimney (d) zonation of Chysomallon squamiferum , Gigantopelta aegis , Bathymodiolus marisindicus , and Neolepas sp. -

Background Document for Oceanic Ridges with Hydrothermal Vents/Fields
Background Document for Oceanic ridges with hydrothermal vents/fields Biodiversity Series 2010 OSPAR Convention Convention OSPAR The Convention for the Protection of the La Convention pour la protection du milieu Marine Environment of the North-East Atlantic marin de l'Atlantique du Nord-Est, dite (the “OSPAR Convention”) was opened for Convention OSPAR, a été ouverte à la signature at the Ministerial Meeting of the signature à la réunion ministérielle des former Oslo and Paris Commissions in Paris anciennes Commissions d'Oslo et de Paris, on 22 September 1992. The Convention à Paris le 22 septembre 1992. La Convention entered into force on 25 March 1998. It has est entrée en vigueur le 25 mars 1998. been ratified by Belgium, Denmark, Finland, La Convention a été ratifiée par l'Allemagne, France, Germany, Iceland, Ireland, la Belgique, le Danemark, la Finlande, Luxembourg, Netherlands, Norway, Portugal, la France, l’Irlande, l’Islande, le Luxembourg, Sweden, Switzerland and the United Kingdom la Norvège, les Pays-Bas, le Portugal, and approved by the European Community le Royaume-Uni de Grande Bretagne and Spain. et d’Irlande du Nord, la Suède et la Suisse et approuvée par la Communauté européenne et l’Espagne. Acknowledgement This report has been prepared by Ricardo Serrão Santos and Ana Colaço (Department of Oceanography and Fisheries of the University of the Azores) for Portugal as lead country. [[email protected]] Photo acknowledgement Cover page: © Missão Seahma. 2002 (FCT/PCDTM 1999MAR/15281) Contents Background document for Oceanic -

A New Caridean Shrimp of the Family Alvinocarididae from Thermal Vents at Menez Gwen on the Mid-Atlantic Ridge
23 April 2003 PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 1I6(1):I58-I67. 2003. A new caridean shrimp of the family Alvinocarididae from thermal vents at Menez Gwen on the Mid-Atlantic Ridge Timothy M. Shank and Joel W. Martin (TMS) Biology Department, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts 02543, U.S.A., e-mail: [email protected]; (JWM) Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, U.S.A., e-mail: [email protected] Abstract.—A new species of alvinocaridid shrimp, Alvinocaris williamsi, is described from the Menez Gwen hydrothermal vent field on the Mid-Atlantic Ridge. The new species is most similar to another relatively shallow water alvinocaridid, A. stactophila Williams, known only from a cold seep area off the coast of Louisiana, but differs in details of the rostrum and appendages. Alvinocaris williamsi is also morphologically similar to two species of the genus recently described from the Mid-Okinawa Trough, Japan. Molecular phy- logenetic studies of A. williamsi, the eighth described member of the genus, may provide significant insights into the role that deep-sea hydrothermal vents at mid-ocean ridges and hydrocarbon seeps on continental margins have played in the evolution of fauna endemic to these chemosynthetic habitats. The caridean shrimp family Alvinocari The type genus of the Alvinocarididae, didae was originally proposed by Christof- Alvinocaris, is the most diverse genus of fersen (1986) to accommodate a number of the family and contains five hydrothermal morphologically similar shrimp known vent-endemic species: A. brevitelsonis Kik- from hydrothermal vents and hydrocarbon uchi & Hashimoto, 2000, from the Minami- seeps. -
Regional Environmental Assessment of the Northern Mid-Atlantic Ridge
Regional Environmental Assessment of the Northern Mid-Atlantic Ridge Document prepared by the Atlantic REMP project to support the ISA Secretariat in facilitating the development of a Regional Environmental Management Plan for the Area in the North Atlantic by the International Seabed Authority Supported by Legal Notice This document has been prepared for the European Commission however it reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein Disclaimer by ISA secretariat: The views expressed are those of the author(s) and do not necessarily reflect those of the International Seabed Authority. Regional Environmental Assessment of the Northern Mid-Atlantic Ridge Authorship The primary authors for each chapter are listed below Geological Overview of the Mid-Atlantic P.P.E. Weaver, Seascape Consultants Ltd Ridge Contract areas and the mining process P.P.E. Weaver, Seascape Consultants Ltd Physical Oceanography of the North Atlantic A.C. Dale, Scottish Association for Marine Science Biology Chapters R. E. Boschen-Rose, Seascape Consultants D.S.M. Billett, Deep Seas Environmental Solutions Ltd A. Colaço, U. Azores D. C. Dunn, Duke University T. Morato, U. Azores I. G. Priede, U. Aberdeen Cumulative impacts D. Jones, NOC D.S.M. Billett, Deep Seas Environmental Solutions Ltd Citation: Weaver, P.P.E., Boschen-Rose, R. E., Dale, A.C., Jones, D.O.B., Billett, D.S.M., Colaço, A., Morato, T., Dunn, D.C., Priede, I.G. 2019. Regional Environmental Assessment of the Northern Mid-Atlantic Ridge. 229 pages This document benefitted from the invaluable reviews of Marina Carreiro-Silva, U. -

Influence of Hydrothermal Activity and Substrata Nature on Faunal Colonization Processes in the Deep Sea
THESE DE DOCTORAT DE L'UNIVERSITE DE BRETAGNE OCCIDENTALE COMUE UNIVERSITE BRETAGNE LOIRE ECOLE DOCTORALE N° 598 Sciences de la Mer et du littoral Spécialité : Ecologie Marine Par Joan M. ALFARO-LUCAS Influence of hydrothermal activity and substrata nature on faunal colonization processes in the deep sea. Thèse présentée et soutenue à Brest le 10 décembre 2019 Unité de recherche : Unité Etude des Ecosystèmes Profonds (EEP) au laboratoire LEP (Ifremer) Rapporteurs avant soutenance : Composition du Jury : Dr François LALLIER Dr Sabine GOLLNER Professeur, Sorbonne Université Tenure Scientist, Royal Netherlands Institute for Sea Research Dr Jeroen INGELS Dr Eric THIEBAUT Research Faculty, Florida State University Professeur, Sorbonne Université Dr Gérard THOUZEAU (Président du Jury) Research Director, CNRS Dr François LALLIER Professeur, Sorbonne Université Dr Jeroen INGELS Research Faculty, Florida State University Dr Jozée SARRAZIN (Directrice de thèse) Cadre de Recherche, Ifremer Brest Invités : Dr Daniela ZEPPILLI Cadre de Recherche, Ifremer Brest Dr Florence PRADILLON Cadre de Recherche, Ifremer Brest Dr Olivier GAUTHIER Maître de Conférence, Université Bretagne Occidentale iii “It is this contingency that makes it difficult, indeed virtually impossible, to find patterns that are universally true in ecology. This, plus an almost suicidal tendency for many ecologists to celebrate complexity and detail at the expense of bold, first-order phenomena. Of course the details matter. But we should concentrate on trying to see where the woods are, and why, before worrying about the individual tree.” John H. Lawton (1999) iv Acknowledgements I would like to thank so much my supervisors Jozée Sarrazin, Florence Pradillon and Daniela Zeppilli for their massive support, advices and patience during all the thesis. -
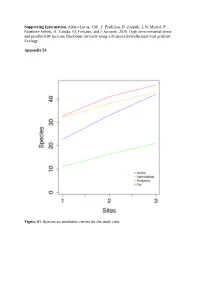
Supporting Information. Alfaro-Lucas, JM, F. Pradillon, D
Supporting Information. Alfaro-Lucas, J.M., F. Pradillon, D. Zeppilli, L.N. Michel, P. Martínez-Arbizu, H. Tanaka, M. Foviaux, and J. Sarrazin. 2020. High environmental stress and productivity increase functional diversity along a deep-sea hydrothermal vent gradient. Ecology. Appendix S4 Figure S1. Species accumulation curves for the study sites. Table S1. Mean abundance (± standard deviation) of taxa on the slate blocks at the four sites. Abundances of Bathymodiolus azoricus are shown, although this species was not included in statistical analyses (see Materials and Methods). Phylum Class Order Species Active Intermediate Periphery Far Annelida Polychaeta Terebellida Amphisamytha lutzi 196,71±247,79 166,78±59,95 0 0 Acrocirridae sp. 0 0,33±0,58 0 0,67±0,58 Flabelligeridae sp. 0 3,56±6,16 0 5,44±7,70 Phyllodocida Glycera tesselata 4,33±6,66 15,67±10,02 6,78±3,27 0,33±0,58 Branchipolynoe seepensis 4,33±2,31 29,33±28,92 0 0 Branchinotogluma sp. 1 0 1,33±2,31 0 0 Bathykermadeca sp. 0 0 0 0,67±1,15 Lepidonotopodium sp. 0,33±0,58 0 0 0,33±0,58 Macellicephala sp. 0,33±0,58 0 0 0 Polynoidae sp. 1 0 0,67±1,15 0 0 Phyllodocidae sp. 0 0 0 0,33±0,58 Tomopteris sp. 1 0,33±0,58 0 0 0 Tomopteris sp. 2 0,33±0,58 0 0 0 Opheliidae sp. 0 0 0 0,33±0,58 Nereididae sp. 0 0 0,33±0,58 0,33±0,58 Spionida Prionospio unilamellata 1,67±2,89 0,33±0,58 0 0 Laonice athecata 0 0,33±0,58 0 0 Eunicia Ophryotrocha cf. -

Exitomelita Sigynae Gen. Et Sp. Nov.: a New Amphipod from the Arctic Loki Castle Vent Field with Potential Gill Ectosymbionts
Heriot-Watt University Research Gateway Exitomelita sigynae gen. et sp. nov.: a new amphipod from the Arctic Loki Castle vent field with potential gill ectosymbionts Citation for published version: Tandberg, AH, Rapp, HT, Schander, C, Vader, W, Sweetman, AK & Berge, J 2012, 'Exitomelita sigynae gen. et sp. nov.: a new amphipod from the Arctic Loki Castle vent field with potential gill ectosymbionts', Polar Biology, vol. 35, no. 5, pp. 705-716. https://doi.org/10.1007/s00300-011-1115-x Digital Object Identifier (DOI): 10.1007/s00300-011-1115-x Link: Link to publication record in Heriot-Watt Research Portal Document Version: Publisher's PDF, also known as Version of record Published In: Polar Biology General rights Copyright for the publications made accessible via Heriot-Watt Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy Heriot-Watt University has made every reasonable effort to ensure that the content in Heriot-Watt Research Portal complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 24. Sep. 2021 Polar Biol (2012) 35:705–716 DOI 10.1007/s00300-011-1115-x ORIGINAL PAPER Exitomelita sigynae gen. et sp. nov.: a new amphipod from the Arctic Loki Castle vent field with potential gill ectosymbionts Anne Helene Tandberg • Hans Tore Rapp • Christoffer Schander • Wim Vader • Andrew K.