Virginia Pediatrics Fall 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non-Incineration Medical Waste Treatment Technologies
Non-Incineration Medical Waste Treatment Technologies A Resource for Hospital Administrators, Facility Managers, Health Care Professionals, Environmental Advocates, and Community Members August 2001 Health Care Without Harm 1755 S Street, N.W. Unit 6B Washington, DC 20009 Phone: 202.234.0091 www.noharm.org Health Care Without Harm 1755 S Street, N.W. Suite 6B Washington, DC 20009 Phone: 202.234.0091 www.noharm.org Printed with soy-based inks on Rolland Evolution, a 100% processed chlorine-free paper. Non-Incineration Medical Waste Treatment Technologies A Resource for Hospital Administrators, Facility Managers, Health Care Professionals, Environmental Advocates, and Community Members August 2001 Health Care Without Harm www.noharm.org Preface THE FOUR LAWS OF ECOLOGY . Meanwhile, many hospital staff, such as Hollie Shaner, RN of Fletcher-Allen Health Care in Burlington, Ver- 1. Everything is connected to everything else, mont, were appalled by the sheer volumes of waste and 2. Everything must go somewhere, the lack of reduction and recycling efforts. These indi- viduals became champions within their facilities or 3. Nature knows best, systems to change the way that waste was being managed. 4. There is no such thing as a free lunch. Barry Commoner, The Closing Circle, 1971 In the spring of 1996, more than 600 people – most of them community activists – gathered in Baton Rouge, Up to now, there has been no single resource that pro- Louisiana to attend the Third Citizens Conference on vided a good frame of reference, objectively portrayed, of Dioxin and Other Hormone-Disrupting Chemicals. The non-incineration technologies for the treatment of health largest workshop at the conference was by far the one care wastes. -

Burn Model System Summary Report
Burn Model System Summary Report 1994–2017 This report contains information, tables, and figures about the data contained in the Burn Model System National Database, collected from 1993 to 2017. The Burn Model System is funded by the National Institute for Disability, Independent Living, and Rehabilitation Research. This report was produced by the BMS National Data and Statistical Center. 2018 Table of Contents Introduction ............................................................................................................................................................................................ 1 Burn Model System Centers ............................................................................................................................................................. 3 Boston-Harvard Burn Injury Model System (BHBIMS) ...................................................................................................... 3 North Texas Burn Rehabilitation Model System (NTBRMS) ........................................................................................... 3 Pediatric Burn Injury Rehabilitation Model System ........................................................................................................... 4 Northwest Regional Burn Model System (NWRBMS) ....................................................................................................... 4 Burn Model System National Data and Statistical Center (BMS NDSC) .................................................................... 5 Summary -

Burn Intensive Care Treatment Over the Last 30 Years: Improved Survival And
b u r n s 4 5 ( 2 0 1 9 ) 1 0 5 7 – 1 0 6 5 Available online at www.sciencedirect.com ScienceDirect jo urnal homepage: www.elsevier.com/locate/burns Burn intensive care treatment over the last 30 years: Improved survival and shift in case-mix a,b, c,d a,e Rolf K. Gigengack *, Margriet E. van Baar , Berry I. Cleffken , a a,c,f Jan Dokter , Cornelis H. van der Vlies a Department of Trauma and Burn Surgery, Maasstad Hospital, Maasstadweg 21, 3079 DZ Rotterdam, The Netherlands b Department of Anesthesiology, Amsterdam UMC, Location VU Medical Center, Boelelaan 1117, 1081HV Amsterdam, The Netherlands c Association of Dutch Burn Centers, Maasstad Hospital, Maasstadweg 21, 3079 DZ Rotterdam, The Netherlands d Department of Public Health, Erasmus MC, Doctor Molewaterplein 40, 3015 GD Rotterdam, The Netherlands e Department of Intensive Care Medicine, Maasstad Hospital, Maasstadweg 21, 3079 DZ Rotterdam, The Netherlands f Trauma Research Unit Department of Surgery, Erasmus MC, Doctor Molewaterplein 40, 3015 GD Rotterdam, The Netherlands a r t i c l e i n f o a b s t r a c t Article history: Purpose: Mortality in burn intensive care unit (ICU) has been decreasing and treatment Accepted 7 February 2019 appears to be changing. The aims of this study: (1) examine outcome in burn patients, (2) examine changes in ICU indication and (3) explore the influence of a changing case-mix. Methods: Retrospective study in patients admitted to ICU (1987–2016). Four groups were specified: major burns (15% TBSA), inhalation injury with small injury (<15% TBSA, < Keywords: inhalation injury), watchful waiting ( 15% TBSA, without inhalation injury), tender loving care (patients withheld from treatment). -

UNIVERSITY of CALIFORNIA, IRVINE Intravenous
UNIVERSITY OF CALIFORNIA, IRVINE Intravenous Acetaminophen: A Non-Narcotic Adjunct for Burn Wound Care THESIS submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE In Biomedical and Translational Science by Sarah Dobson Thesis Committee: Professor Sherrie H. Kaplan, Chair Assistant Clinical Professor Nicole P. Bernal Associate Clinical Professor Steven Mills 2014 © 2014 Sarah Dobson TABLE OF CONTENTS Page LIST OF FIGURES………………………………………………………...………………….v LIST OF TABLES…………………………………………………………..…….……………vi ACKNOWLEDGEMENTS…………………………………………………………………….vii ABSTRACT OF THE THESIS…………………………………………...………….............viii CHAPTER 1: INTRODUCTION………………………………………………..…………….1 CHAPTER 2: BACKGROUND………………………………………………………...……..3 Burn Wound Care…………………………………………………………….............3 Background versus Breakthrough Pain……………………………………............3 Narcotic Pain Medications…...…………………………………………..…………..4 Intravenous Acetaminophen…………………………………………...…………….5 First-pass Metabolism………………………………………………..……………....8 CHAPTER 3: METHODS……………………………………………………………….……9 Setting…………………………………………………………………..………...……9 Sample…………………………………………………………………………………9 Exclusion Criteria…………………………………………………………………….11 Study Design……………………………………………………………………....…13 Pain Measures…………………………………………………………………….…14 Outcome Measures………………………………………………………………….15 Organizing the Data……………………………………………...………………….16 Analyzing the Data………………………………………………...…………..…….17 Power Analysis…………………………………………………………………….…18 iii CHAPTER 4: RESULTS……………………………………………………….……………19 Demographics…………….……………………………………………..……...……19 -

Copyrighted Material
Index Note: Page references in italics refer to Figures; those in bold refer to Tables 1% rule 92–3 endotracheal intubation 24–5 asthma 27, 28, 31, 126–7, 126, 127 30-second drills 25, 48, 48 supraglottic airway devices (SADs) 23–4, 24 Australasia, training in PHEM in 1–2 airway obstruction, causes 20 automatic vehicle location system (AVLS) 4 ABC system 18, 18, 18 alcohol gel 9 AVPU (alert, voice, pain, unresponsive) score 60 ABCDEF approach 151 alkali burns 90–1, 91 abdominal injury 81–3 altitude injury 141–3 bag-valve-mask (BVM) 29–30, 30, 161 essential drugs 143 analgesia 82 ballistic injuries 76, 103 physiology at altitude 141 assessment 82 abdominal 81 Alzheimer’s disease 166 blunt trauma 81 airway management 107, 107 American National Standards Institute 9 in children 163 breathing management 107–8 amputation 87–8, 88–9, 89 evisceration 82, 82 catastrophic haemorrhage control 106–7 blast/ballistic 108 extent of abdomen 82, 82 cervical spine immobilization 107 crush injury 101 impalement 83, 83 circulation management 108 anaesthesia, prehospital 44–50 mechanisms 81 disability 108 30-second drills 48, 48 penetrating trauma 81 exposure 108 clinical assessment 45 prehospital management 82–3 firearm injuries 103 drugs 45–6, 46 resuscitation 82 management 106–8, 106 equipment checklist 47, 47 triage 83 ballistic jacket 105, 105 evidence base 44–5 abuse bariatric ambulance 171 general principles 46 child 164, 167 guidelines 44–5 bariatric patient 168–71 elderly 167 indications 44 barotrauma 147 acclimatization 141, 141 induction and intubation -

Basic Eye Anatomy Cross ‐ Section View of the Anterior
Sound like an expert, learn the lingo… First: basic eye anatomy •Anatomical Landmarks • Cornea External/ .Lids/Lashes • Iris Ocular .Lacrimal system • Adnexa Lens .Conjunctiva and Sclera • Disc– meaning optic nerve • Fundus– meaning retina .Cornea Anterior . •Common Abbreviations for Common Conditions: Segment Anterior Chamber • POAG‐ primary open angle glaucoma .Iris • PDR‐ proliferative diabetic retinopathy .Lens • AMD (ARMD) – age‐related macular degeneration • DME‐ diabetic macular edema .Vitreous Posterior • phaco‐ phacoemulsification (aka: cataract surgery) and typically this would include an IOL Segment .Optic nerve • IOL‐ intraocular lens .Retina • IOP‐ intraocular pressure • DES‐ dry eye syndrome • TED‐ thyroid eye disease https://www.aao.org/young‐ophthalmologists/yo‐info/article/learning‐lingo‐ophthalmic‐abbreviations 7 8 Cross ‐ Section view of the anterior segment Basic anatomy Space between cornea and iris Anterior (translucent covering) Segment Posterior Segment 9 10 Syllabi/Slides for this program are a supplement to the live CME session and are not intended for other purposes. Your patient has an eye complaint, now what? But wait, don’t I need these? History: ◦ Laterality‐ is it one eye or both eyes? ◦ Any recent eye surgery or trauma? ◦ Ask about RSVP symptoms ‐ Redness, Sensitivity to light, Vision loss, Pain Vision: ◦ Check one eye at a time with near card with patient’s glasses on Motility: ◦ Have the patient move their eyes up and down, then side to side ◦ Do both eyes move together in the same direction? Penlight Exam: ◦ Conjunctiva and sclera ◦ Cornea and anterior chamber ◦ Pupil size, shape, reaction, and color 11 12 Basic Tool Kit Allow the patient to hold the card at a comfortable reading distance. -

Table of Contents ELJ 171.Indd
Egypt J. Med. Lab. Sci., April. 2008; 17(1):35-43 ISSN 1110-5593 METHICILLIN RESISTANT STAPHYLOCOCCUS AUREUS AS A NOSOCOMIAL PATHOGEN IN BURN WOUND INFECTION Original Nehal I. Draz, Mahmoud K. Elsayed1, and Hazem M. Aly2 Article 1Microbiology, immunology. and 2Plastic surgery departments, Faculty of medicine, Ain Shams University ABSTRACT Background: Methicillin resistant staphylococcus aureus (MRSA) is important as a nosocomial pathogen and MRSA colonization in the burn unit may lead to increased transmission of MRSA to non burn patients in other wards. Aim of the Work: was to determine the prevalence of MRSA among patients with burn wound infection in the Burn Unit in Ain Shams University Hospitals. Patients and Methods: The study involved fifty patients with infected burn wounds divided into two groups as twenty five outpatients and twenty five inpatients in the Burn Unit of Ain Shams University Hospital. Swabs were taken aseptically from infected wounds for bacteriological examination. Results: The most common bacterial isolate among outpatients group was S. aureus (44%), followed by Ps. aeurginosa (40%), coagulase negative Staphylococci (12%), Proteus mirabilis (4%) and Citrobacter freundii (8%). While in the inpatients group, Ps. Aeruginosa topped the list infecting 36% of patients, followed by S. aureus (24%), coagulase negative Staphylococci (16%), Klebsiella pneumoniae (12%), E.coli (12%), Proteus mirabilis (8%) and S. pyogenes (4%). Concerning MRSA, it was isolated from 20% of the inpatients, accounting for 83% of all isolated S.aureus and emerged as a significant nosocomial pathogen as it was not recovered from any outpatient. Conclusion: MRSA is a significant nosocomial pathogen in the Burn unit of Ain Shams University Hospital. -

Tintinalli – Electrical and Lightning Injuries
6/11/2019 Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 8e Chapter 218: Electrical and Lightning Injuries Caitlin Bailey INTRODUCTION Electrical injuries are divided into high-voltage injuries (≥1000 V), low-voltage injuries (<1000 V), and electric arc flash burns, which by definition do not result in passage of current through the tissues. Lightning injury is an extreme and unique form of electrical injury. This chapter also discusses injuries caused by electronic control devices, such as the Taser®. Burns from electrical accidents can result from heating due to electric current flow through tissues, explosions, and burning of flammable liquids, clothes, and other objects. Burns are discussed in the chapter 216, "Thermal Burns." EPIDEMIOLOGY Approximately 6500 electrical injuries occur per year in the United States, accounting for 4% of total burns. Of these, the majority are work related (61%), mostly industrial injuries. The overall complication rate is 10.6%, with the fewest complications among children age 1 to 5 years (2%).1 The most common high-voltage injuries in the United States are also work related and include arc burns in electricians and high-voltage injuries in power line workers.2 The Electrical Safety Foundation International estimates that contact with electric current caused nearly 1800 workplace fatalities between 2003 and 2010.3 High-voltage power line injuries are particularly disabling because they oen lead to deep-muscle necrosis and the need for fasciotomy and amputation.2 BASICS OF CURRENT FLOW Electric current is the movement of electrical charges. Table 218-1 lists a few key terms related to electricity. 1/33 6/11/2019 TABLE 218-1 Electrical Terms and Units of Measure Term Unit of Measure Electric current Amperes Movement of electrical charges Current flow Volts Driven by voltage or electrical potential dierence Resistance Ohms Hindrance to flow of current Ohm's law (current = voltage/resistance) — The current is proportional to voltage. -

Predictors of Inhalation Injury in Burn‐Injured Patients Before Arrival At
Emergency Medicine Australasia (2020) doi: 10.1111/1742-6723.13604 ORIGINAL RESEARCH To intubate or not to intubate? Predictors of inhalation injury in burn-injured patients before arrival at the burn centre Kylie DYSON ,1,2 Paul BAKER,3 Nicole GARCIA,3 Anna BRAUN,3 Myat AUNG,4 David PILCHER,4 Karen SMITH,2 Heather CLELAND3,5 and Belinda GABBE1,6 1Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Victoria, Australia, 2Centre for Research and Evaluation, Ambulance Victoria, Melbourne, Victoria, Australia, 3Victorian Adult Burns Service, Alfred Hospital, Melbourne, Victoria, Australia, 4Intensive Care Unit, Alfred Hospital, Melbourne, Victoria, Australia, 5Central Clinical School, Monash University, Melbourne, Victoria, Australia, and 6Health Data Research UK, Swansea University Medical School, Swansea, UK Abstract injury was 11%. Increasing %TBSA Key findings burned, flame, enclosed space, face Objective: Inhalation injury occurs burns, hoarse voice, soot in mouth and • Eleven percent of burn-injured in approximately 10–20% of burn shortness of breath were predictive of patients in our study had con- patients and is associated with inhalation injury. The model provided firmed inhalation injury. increased mortality. There is no clear excellent discrimination (area under • The mortality rate of patients method of identifying patients at risk fi curve 0.87, 95% con dence interval with inhalation injury was of inhalation injury or requiring – 0.84 0.91). A lower proportion of 16%. intubation in the pre-hospital setting. patients intubated at a non-burn centre • Increasing percentage of total Our objective was to identify pre- had an inhalation injury (33%) com- burn centre factors associated with pared to patients intubated by emer- body surface area burned, fl inhalation injury confirmed on bron- gency medical services (54%) and in the ame, enclosed space, face choscopy, and to develop a prognos- burn centre (58%). -

K Allison, K Porter. Consensus on the Pre-Hospital Approach to Burns
J R Army Med Corps 2004; 150: 10-13 J R Army Med Corps: first published as 10.1136/jramc-150-01-02 on 1 March 2004. Downloaded from Consensus On The Pre-hospital Approach To Burns Patient Management K Allison, K Porter Introduction children and there is a lack of teaching and In the United Kingdom (UK), burns simple, evidence-based guidelines. patients account for approximately 175,000 The Faculty of Pre-hospital Care set out to emergency department attendances and improve the information available concerning 15,000 hospital admissions each year (1). immediate care of the burns patient in simple, Consequently the first aid and pre-hospital unambiguous guidelines, so that any carer care for this large group of patients is of great (including first-aider, ambulance personnel, importance and yet in the authors’ nurse or doctor) could administer safe, experience, simple things are often not done appropriate care. The process to achieve very well. consensus over these guidelines has taken In 1998 a national survey revealed 58% of time and the advice and ratification by all UK ambulance services had no specific groups that look after burns patients from the treatment policy for burns patients (2). Pre- point of their injury through to definitive care hospital carers often feel out of their depth in has been painstakingly followed (Tables caring for burns patients, particularly 1&2). Table 1.Process of consensus guidelines. Ambulance service and plastic & burns surgeons 1998 questionnaire survey Presentation of data at Trauma UK meeting June 1999 Presentation of Data at British Burns Association April 2000 (BBA) meeting Letter in BBA newsletter inviting suggestions and September 2000 help http://militaryhealth.bmj.com/ Publication: “The UK pre-hospital management of Burns 2002; 28:135-42. -
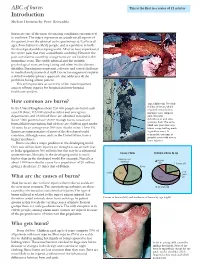
ABC of Burns Introduction
Downloaded from bmj.com on 30 September 2006 Clinical review ABC of burns This is the first in a series of 12 articles Introduction Shehan Hettiaratchy, Peter Dziewulski Burns are one of the most devastating conditions encountered in medicine. The injury represents an assault on all aspects of the patient, from the physical to the psychological. It affects all ages, from babies to elderly people, and is a problem in both the developed and developing world. All of us have experienced the severe pain that even a small burn can bring. However the pain and distress caused by a large burn are not limited to the immediate event. The visible physical and the invisible psychological scars are long lasting and often lead to chronic disability. Burn injuries represent a diverse and varied challenge to medical and paramedical staff. Correct management requires a skilled multidisciplinary approach that addresses all the problems facing a burn patient. This series provides an overview of the most important aspects of burn injuries for hospital and non-hospital healthcare workers. How common are burns? Top: Child with 70% full thickness burns, which In the United Kingdom about 250 000 people are burnt each required resuscitation, year. Of these, 175 000 attend accident and emergency intensive care support, departments, and 13 000 of these are admitted to hospital. and extensive Some 1000 patients have severe enough burns to warrant debridement and skin grafting. Left: The same formal fluid resuscitation; half of these are children aged under child one year later at a 12 years. In an average year 300 burn deaths occur. -

The Burn Injury Model System Centers Program
The Burn Model System Centers Program A project funded by the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR), A Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS) 1 Project Design • A unique prospective, longitudinal multi-center study • Examines health, employment, and community reintegration outcomes post-burn injury • Consists of the BMS National Longitudinal Database as well as site-specific and collaborative projects 2 Burn Model System (BMS) Program Design • One of three multi-center programs sponsored by NIDILRR to examine outcomes following the delivery of a coordinated system of acute trauma care and rehabilitation: • Burn Injury Model System (begun 1994) • Traumatic Brain Injury Model System (begun 1987) • Spinal Cord Injury Model System (begun 1970) • Examines the course of recovery and the health, employment, and community reintegration outcomes after burn injury • Consists of: • Prospective, longitudinal national database • Site-specific research projects • Multi-center research and knowledge translation projects 3 Increase in Survival Advances in acute burn care have significantly reduced mortality and increased the number of people living with severe burn injury. Tompkins RG. Survival from Burns in the New Millennium: 70 Years Experience from a Single Institution. Annals of Surgery. 2015;261(2):263-268. 4 As a result of major decreases in mortality, the field of burn care has transformed and is increasingly focused on improving the quality of life and long term outcomes of burn survivors. 5 Long-term Outcomes • The BMS is the only multi-center, research program dedicated to advancing the long-term recovery of burn survivors.