CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Branch Libraries List
ADDRESS OF BRANCH LIBRARIES 1 District Central Library, 16 Branch Library, 307, Anna Salai, 2D, Nadu Street, Chengalpet – 603 002. Achirupakkam – 603 301. 2 Branch Library, 17 Branch Library, 78, Station Road, Main Road, Kattangolathur – 603 203. Thozhupedu – 603 310. 3 Branch Library, 18 Branch Library, Gandhi Street, Main Road, Guduvancheri – 603 202. Orathy – 603 307. 4 Branch Library, 19 Branch Library, 2/45, B. Santhaimedu, Ladakaranai, Endathur, Singaperrumal Koil – 603 204. Uthiramerur – 603 406. 5 Branch Library, 20 Branch Library, 129, Thiruvalluvar Salai, Bajanai Koil Street, Maraimalai Nagar – 603 209. Elapakkam – 603 201. 6 Branch Library, 21 Branch Library, 5, West Mada Street, 5/55, Salt Road, Thiruporur – 603 110. Cheyyur – 603 202. 7 Branch Library, 22 Branch Library, 34, Mamallapuram Salai, Angalamman Koil Street, Thirukazhukundram – 603 109. Kuvathur – 603 305. 8 Branch Library, 23 Branch Library, 203, Kulakarai Street, 2, East Coast Road, Sembakkam – 603 108. Kadapakkam – 603 304. 9 Branch Library, 24 Branch Library, 105, W2, Brahmanar Street, 9, Chakkaram Kodhandarama P.V. Kalathur – 603 405. Iyengar Street, Uthiramerur – 603 406. 10 Branch Library, 25 Branch Library, East Raja Street, Hospital Road, Mamallapuram – 603 104. Kaliyampoondi – 603 403. 11 Branch Library, 26 Branch Library, Nesco Joint, 1/172, Road Street, Kalpakkam – 603 102. Manampathi – 603 403. 12 Branch Library, 27 Branch Library, 70, Car Street, Main Road, Madhuranthagam – 603 306. Perunagar – 603 404. 13 Branch Library, 28 Branch Library, 3, Othavadai Street, Perumal Koil Street, Karunguzhi – 603 303. Salavakkam – 603 107. 14 Branch Library, 29 Branch Library, Railway Station Road, 138, Pillaiyar Koil Street, Padalam – 603 308. -

ANSWERED ON:11.05.2005 AUTOMATIC and MODERN TELEPHONE EXCHANGES in TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy
GOVERNMENT OF INDIA COMMUNICATIONS AND INFORMATION TECHNOLOGY LOK SABHA UNSTARRED QUESTION NO:6879 ANSWERED ON:11.05.2005 AUTOMATIC AND MODERN TELEPHONE EXCHANGES IN TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy Will the Minister of COMMUNICATIONS AND INFORMATION TECHNOLOGY be pleased to state: (a) the details of automatic and modern telephone exchanges set up in Tamil Nadu during the last three years, location- wise; (b) the details of such exchanges proposed to be set up in Tamil Nadu during the current year; (c) the details of the telephone exchanges whose capacities were expanded in the current financial year; and (d) the details of telephone exchanges where waiting list for telephone connection still exists? Answer THE MINISTER OF STATE IN THE MINISTRY OF COMMUNICATIONS ANDINFORMATION TECHNOLOGY (DR. SHAKEEL AHMAD) (a) The details of automatic and modern telephone exchanges set up in Tamilnadu during the last three years are given in the Annexures- I(a), I(b) & I(c). (b) The details of such exchanges proposed to be set up in Tamilnadu during the current year are given in Annexure-II. (c) The details of the telephone exchanges whose capacities were expanded in the current financial year are given at Annexure-III. (d) The details of telephone exchanges where waiting list for telephone connection still exists are given in Annexure- IV. ANNEXURE-I(a) DETAILS OF TELEPHONE EXCHANGES SET UP DURING 2002-03 IN TAMILNADU Sl Name of Exchange Capacity Type/Technology District No.(Location) 1 Avinashi-II 4000 CDOTMBMXL Coimbatore 2 K.P.Pudur -

Linkages -3.7.2
3.7.2 Number of linkages with institutions/industries for internship, on-the-job training, project work, sharing of research facilities etc. during the 2014-20 Name of the partnering institution/ industry /research lab with Duration (From- S. No Title of the linkage Year of commencement Nature of linkage Name of the participant Link to document contact details to) Ernst&young LLP 07 January 437, Manapakkam, Chennai, 1 Internship 2018 to 2019 2019 to Student Internship Mr. N. Krishna Sagar http://bit.ly/2TQ3tEX Tamil Nadu 600125 05 April 2019 Phone: 044 6654 8100 Peritus solutions private limited/No.2, 1st Floor, Third Street, Sri 02 January 2 Internship Sakthi Vijaylakshmi Nagar, Off 100 Feet Bypass Road, Velachery 2018 to 2019 2019 to Student Internship Mr.MOHAMMED ZIYYAD A http://bit.ly/3ayUNZr - Chennai - 600 042, Tamil Nadu, Phone: +91 44 48608788 02 April 2019 National Payments Corporation of India 1001A, B wing, 10 Floor, 04 June 2018 3 Summer Internship The Capital, Bandra-Kurla Complex, Bandra (East), Mumbai - 400 2018 to 2019 to Student Internship C.Pooja Priyadarshini http://bit.ly/2vhcM6E 051 Phone - 022 4000 9100 04 August 2018 SIDSYNC Technologies Pvt Ltd/Spaces.Express Avenue EA 24 January Chambers tower II, No. 49/50L,, Whites Road, Royapettah, 4 Internship 2018 to 2019 2019 to Student Internship Mr.JOSHUA J http://bit.ly/2TPUDqI Chennai, Tamil Nadu 600002 24 April 2019 Phone: 098948 19871 TAP Turbo Engineers Private Limited, Ambattur, 20 Jan 2019 5 Internship Chennai 600 58 2018 to 2019 to Student Internship Ms. Sai Gayathri Mahajan http://bit.ly/2uollMu Contact: 0442625 7234 20 March 2019 Trail Cloud Innovation Services Pvt Ltd, 187, Square Space 19 Nov 2018 Business Center, 188, Thiruvalluvar Rd, Block 10, Panneer Mr. -

Prefix Gpfno Name 06/96 04/98 09/13 07/03 02/12 04/93 01/15 08/10 11/91 11/09 03/99 09/91 07/91 09/92 07/91 10/91 03/97 05/13 01
Subscribers with one or two missing credits upto February 2015 18-JAN-21 05:32 PM (Press Ctrl+F and type your gpf number to identify your record in this list) Send recovery amount of subs/refund and month of encashment for clearance of missing credit(s) PREFIX GPFNO NAME DRAWING AND DISBURSING OFFICER MISSING MONTHS AGRI 23136 SENTHIL M SUPDT ENGR AE)LAND DEVELOPMENT O/O THE CHIEF ENGR(AE) CH 35 06/96 , 27131 JOHN DURAIRAJ S ADMN OFFICER O\O THE JOINT DIR OF AGRICULTURE TIRUVALLUR 04/98 , 09/13 , 27365 BALAN S DY DIR OF AGRI WATER MGT TRAINING CENTRE VINAYAGAPURAM MDU 07/03 , 28080 PARTHIBAN K ASST DIR OF AGRI SARKARSAMAKULAM COIMBATORE DIST 02/12 , 28216 AGALYASAMUNDESWARI C JOINT DIRECTOR OF AGRI COIMBATORE 04/93 , 28244 SELVARAJU S ASST DIR OF AGRI PAVINJUR KANCHEEPURAM DIST 01/15 , 08/10 , 28620 VINAYAGAMOORTHY K ASST DIR OF AGRI RAJAPALAYAM 11/91 , 28651 MOHAN DOSS M ASST EXE ENGR (AE) TANJORE 11/09 , 28748 RAVICHANDAR A K ASST EXE ENGR (AE) KUMBAKONAM 03/99 , 09/91 , 28820 MANI R ASST DIR OF AGRI MELBHUVANAGIRI CUDDALORE DIST 07/91 , 09/92 , 28832 BALASUBRAMANIAN T ASST DIR OF AGRI VALANGAIMAN TIRUVARUR 07/91 , 10/91 , 28957 EMMANUEL RAJAA D DY DIR OF AGRI PA TO COLLECTOR (AGRI) COIMBATORE 03/97 , 05/13 , 28960 SAMPATH KUMAR V ADMN OFFICER DIR OF AGRL CHEPAUK CHENNAI - 5 01/95 , 11/91 , 29126 CHANDRANAGAR T DY DIR OF AGRI (AGRI BUSINESS) VIRUDHUNAGAR 11/91 , 29258 RAMASWAMY K ASST EXE ENGR (AE) KRISHNAGIRI 05/93 , 29317 DEVARAJ I ASST EXE ENGR AGRL ENGINEERAING DEPT UDUMALPET TIRUPPUR DIST 09/00 , 29447 KUMARASAMI D DY DIR OF AGRI -

Chengalpattu District
DISTRICT DISASTER MANAGEMENT PLAN 2020 CHENGALPATTU DISTRICT District Disaster Management Authority Chengalpattu District, Tamil Nadu DISTRICT DISASTER MANAGEMENT PLAN 2020 DISTRICT DISASTER MANAGEMENT AUTHORITY CHENGALPATTU DISTRICT TAMIL NADU PREFACE Endowed with all the graces of nature’s beauty and abundance, the newly created district of Chengalpattu is a vibrant administrative entity on the North eastern part of the state of Tamil Nadu. In spite of the district’s top-notch status in terms of high educational, human development index and humungous industrial productivity, given its geography, climate and certain other socio-political attributes, the district administration and its people have to co-exist with the probabilities of hazards like floods, cyclone, Tsunami, drought, heat wave, lightning and chemical, biological, radiological and nuclear emergencies. The Disastrous events in the recent past like the Tsunami of 2004, the catastrophic floods of year 2015, the cyclone of year 2016 and most recently the COVID-19 pandemic, will serve as a testament to the district’s vulnerability to such hazards. How the society responds to such vagaries of nature decides the magnitude and intensity of the destruction that may entail hazardous events. It is against this back drop, the roll of the District Disaster Management Authority can be ideally understood. The change in perspective from a relief- based approach to a more holistic disaster management approach has already begun to gain currency among the policy makers due to its substantial success in efficient handling of recent disasters across the globe. The need of the hour, therefore, is a comprehensive disaster management plan which is participative and people-friendly with the component of inter- departmental co-ordination at its crux. -

Annexure-District Survey Report
3/6/2017 Home TamilNadu Map Cuddalore District Profile Print CUDDALORE DISTRICT PROFILE • Cuddalore district is bounded by Villuppuram district in the north and northwest, Perambalur district in the southwest, and Ariyalur and Nagapattinam districts in the South and Bay of Bengal in the east. • Cuddalore district lies between 11º09'00’’N to 11º53'00’’ N Latitude, 78º52'00’’E to 79º51’00’’E Longitude and has an areal extent of 3706 sq.km • There are 13 Blocks, 683 Villages and 3639 Habitations in the District. Physiography and Drainage: • Cuddalore district being a coastal zone is mostly covered by plain terrain, without any high relief zone except some sedimentary high ground in Virudhachalam, Cuddalore and Panruti blocks. Rainfall: : Acutal Rainfall in mm Normal Rainfall in mm 2011 2012 2013 2014 2015 1461.4 883.20 977.7 1218.1 1705.7 1206.7 Geology: Rock Type Geological Formation Sandstone, Conglomerate, Sedimentary Rock 90% Gneiss, Charnockite, Marine Hard Rock 10 % deposits and Alluvium Hydrogeology: Type of aquifer Fairly thick but discontinuous confined to semi confined aquifers. Alluvium Tertiary Hard rock Aquifer parameters Well yield in lpm 150 Hard rock 60120 Transmissivity (T) m 2 /day 98 46134 16160 Permeability (K) m/day 19.7 1633 5 – 20 Sp. capacity. lpm/md 208 78.17 27224 Ground Water Level:: The Ground Water levels from the 42 number of observation wells of TWAD have been analysed for PostMonsoon and Pre Monsoon. Since 1991, average Ground water level in m Below Ground Level for pre and post monsoon is as follows: 1/3 3/6/2017 Sustainability:: With a view to enhance the Sustainability of the drinking water sources, recharge structures are being implemented by TWAD Board under various State and Central Government assistances. -

Pre Matric Scholarship 2019-2020 - Fresh Name / Father Sl
Pre Matric Scholarship 2019-2020 - Fresh Name / Father Sl. no Applicant Id Institute name Address Disb.Amt Name J.M.HR.SEC.SCH., BLOCK 28 ( CUDDALORE - 3/5 I ST MAIN STREET BLOCK 28 1 TN201920005436455 SHRROFINA /JAMES A TAMIL NADU ) / 33180400819 NEYVELI TOWNSHIP 5200 NO 12/17 NORTH STREET J.M.HR.SEC.SCH., M.KUPPAM ( CUDDALORE - VELIKUNANKUIRCHI, 2 TN201920003402060 AARLIN /SOWRIRAJ TAMIL NADU ) / 33180400820 OOMANGALAM PO 1000 12/17 NORTH STREET J.M.HR.SEC.SCH., M.KUPPAM ( CUDDALORE - VELIKOONANKURICHI, 3 TN201920003395417 ARNALD /SOWRIRAJ TAMIL NADU ) / 33180400820 UMANGALAM 5200 TONI JACKSON /ANTONI JAWAHAR CBSE PRIMARY SCHOOL BL-24 ( NO 473/B, ARUL IRUKKUM, 4 TN201920007584045 RAJ CUDDALORE - TAMIL NADU ) / 33180400827 PANIKKANKUPPAM 1000 JAWAHAR CBSE PRIMARY SCHOOL BL-24 ( 7/3c, thenkuthu road, 5 TN201920003912692 SAMUEL B /D baskar CUDDALORE - TAMIL NADU ) / 33180400827 abatharana puram, vadalur 1000 KALVIN ABISHAK DANY A JAWAHAR CBSE PRIMARY SCHOOL BL-24 ( MIDDLE STREET, TENKUTHU, 6 TN201920008879111 /ANTONY RAJ S CUDDALORE - TAMIL NADU ) / 33180400827 VANATHIRYAPURAM 1000 FATHINA R JAWAHAR CBSE PRIMARY SCHOOL BL-9 ( 9 FATHIMA COLONY,OLD 7 TN201920007679671 /RASHEEKAPOOR CUDDALORE - TAMIL NADU ) / 33180400824 NEYVELI, NEYVELI 2 1000 JAWAHAR METRIC B.MUTLUR ( CUDDALORE 264, JAMAL MOHAMED STREET, 8 TN201920003861511 ARSHANA /SHAIK ISMAIL - TAMIL NADU ) / 33181000804 B.MUTLUR. 1000 JASMINA FARVEEN HAJA NAJIMUDEEN /HAJA JAWAHAR METRIC B.MUTLUR ( CUDDALORE 394, KODIKKAL NAGAR, B. 9 TN201920002594020 NAJIMUDEEN - TAMIL NADU ) / 33181000804 MUTLUR 5200 AYEESHA SIDDIKA JAWAHAR METRIC B.MUTLUR ( CUDDALORE 439, KODIKAL NAGAR, B. 10 TN201920004836529 /ABDUL WADOOD - TAMIL NADU ) / 33181000804 MUTLUR 5200 JAWAHAR METRIC B.MUTLUR ( CUDDALORE 11 TN201920002220814 SHAFREEN /ISMAIL - TAMIL NADU ) / 33181000804 140/7, Main Road, B.Mutlur. -

Post Matric Scholarship 2019-20 (Fresh) - Panruti Taluk Name / Father Disb
Post Matric Scholarship 2019-20 (Fresh) - Panruti Taluk Name / Father Disb. Sr no Applicant Id Institute name Address Name Amt SAHANANASRIN A ADWGHSS DEEVALORE ( CUDDALORE - TAMIL 82, ROAD STEERT, SATHUKUDAL 1 TN201920002982152 /AKBARALI NADU ) / 33180703003 MELPATHI, 2300 MAJU BEGAM /Haja AHSS GIRLS CHRISTIAN , MELPATTAMPAKKAM 73,Muslim Street 2 TN201920003306782 Kamal ( CUDDALORE - TAMIL NADU ) / 33180202505 Melpattambakkam 3300 HAJIRA BANU AHSS GIRLS CHRISTIAN , MELPATTAMPAKKAM 26,Muslim 3 TN201920006225950 /Mohammed Farook ( CUDDALORE - TAMIL NADU ) / 33180202505 Street,Melpattambakkam 2300 AMARSHIYA ALANGARA ANNAI GIRLS.HSS.VARAD ( 857/3,ANTHONIYARPURAM,KOZHA 4 TN201920007668472 /RATCHAGAR ARIYALUR - TAMIL NADU ) / 33160802305 I 6300 ALANGARA ANNAI GIRLS.HSS.VARAD ( 5 TN201920007611657 A SECILI /ANOKE ARIYALUR - TAMIL NADU ) / 33160802305 936,ANTHONIYARPURAM,KOZHAI 6300 JANCI RANI A /AROKIA ANNAI LOURD GIR.HSS THENNUR ( ARIYALUR - 181/1,MADHAKOVIL 6 TN201920007774945 DOSS TAMIL NADU ) / 33160804303 ST,VANAMADEVI 6300 ANNAI VAILANKANNI COLLEGE OF ARTS AND SCIENCE, V.O.C. NAGAR, THANJAVUR - 613 600 , R C STREET, SAVADIKUPPAM 7 TN201920001154965 SANTHIYA K /KASBAR A 007. ( THANJAVUR - TAMIL NADU ) / 40866 ,KOZHAI (PO),CUDDALORE 6000 ANNIESHIRLIN T BESCHI HSS ULUNDUPET ( VILLUPURAM - 8 TN201920000186025 /Thomas Alva Edison TAMIL NADU ) / 33072107505 30, OLD COLONY, M.PARUR 10800 C.KANDASWAMI NAIDU COLLEGE FOR SHARMILA BEGAM L WOMEN,CUDDALORE-607001 ( CUDDALORE - 9 TN201920008540246 /liyagathali khan TAMIL NADU ) / 41058 4th Street police -

The Hon'ble Mr.Justice C.T.Selvam Orders to Be
THE HON'BLE MR.JUSTICE C.T.SELVAM ORDERS TO BE DELIVERED ON MONDAY THE 13TH DAY OF JULY 2015 AT 2.00 P.M. (SITTING IN HIS LORDSHIP'S CHAMBERS) ----------------------------------------------------------------------------------------- FOR ORDERS ~~~~~~~~~~~~ (ORDERS WERE RESERVED DURING HIS LORDSHIP'S SITTING IN THE MADURAI BENCH OF MADRAS HIGH COURT AT MADURAI) TO RECALL THE ORDER 1. MP(MD).1/2014 M/S. M. KARUNANITHI PUBLIC PROSECUTOR FOR R1 S. RAJAPRABU M/S P.SENGUTTARASAN K.SIVAKUMAR FOR PETITIONER IN CRL OP. in CRL OP(MD).13457/2013 ****************************** THE HON'BLE MS. JUSTICE K.B.K. VASUKI TO BE HEARD ON MONDAY THE 13TH DAY OF JULY 2015 AT 1.45 P.M. (SITTING IN HER LORDSHIP'S CHAMBERS) -------------------------------------------------------------------------------------------- ---- FINAL HEARING CASES ~~~~~~~~~~~~~~~~~~~ PART HEARD 1. CRP.1601/2008 M/S.K.GOVI GANESAN CRP.1601/2008 M/S.R.MOHAN S.SARAVANAN FOR SOLE RESPT CRP.4771/2013 M/S.S.SARAVANAN FOR R1 R2-BANK OF MAHARASHTRA REP BY ITS BRANCH MANAGER NO.3 NAGESWARA RAO ROAD T.NAGAR CHENNAI 600 017 and For Stay MP.1/2008 - DO - and To permit MP.1/2013 - DO - and CRP.4771/2013 - DO - ***************( Concluded )*************** THE HON'BLE MR JUSTICE M. VENUGOPAL TO BE HEARD ON MONDAY THE 13TH DAY OF JULY 2015 AT 1.45 P.M. (SITTING IN HIS LORDSHIP'S CHAMBERS) ------------------------------------------------------------------------------------------- MISCELLANEOUS PETITIONS ~~~~~~~~~~~~~~~~~~~~~~~ 1. CONT P.131/2015 M/S.P.K.RAJAGOPAL MR.I.AROCKIASAMY D.AROKIA MARY SOPHY GOVT.ADVOCATE NOTICE SENT SERVICE AWAITED ***************( Concluded )*************** LOK ADALAT I ~~~~~~~~~~~~ PRESIDED OVER BY THE HON'BLE MR.JUSTICE MALAISUBRAMANIAN (Retd.) TO BE HEARD ON MONDAY THE 13TH DAY OF JULY 2015 AT 11.00 A.M. -

Guduvancheri, Chennai
® Guduvancheri, Chennai The Emerging Suburb of South Chennai Micro Market Overview Report February 2018 About Micro Market Located in the peripheral areas of south-western Road is easily accessible from the micro market via quadrant of Chennai, Guduvancheri is emerging as Kelambakkam – Vandalur Road. one of the prominent real estate hotspots. The micro The micro market has a well-developed social market is located alongside National Highway-45 infrastructure with the presence of several (NH-45), which is also known as the Grand Southern educational institutes such as the SRM School of Trunk Road (GST Road). Surrounded by lakes, Hotel Management, Bharathiyar Matriculation Guduvancheri is located between Urapakkam and Higher Secondary School, Saint Mary’s Matriculation Potheri. Guduvancheri’s calm and serene School and Guduvancheri Boys Higher Secondary environment has attracted several developers to School. In addition, the micro market houses several build residential complexes in this region. The micro healthcare centers such as Christudas Hospital, Sri market is surrounded by busy areas such as Venkateswara Clinic, Vasantham Clinic, K. R. Nursing Maraimalai Nagar and Adhanur. Guduvancheri lies in Home and Sumana Goodwill Home. Guduvancheri proximity to a multitude of IT Parks/SEZs such as also has good retail options with presence of Shriram The Gateway and Mahindra World City SEZ. shopping complexes such as S.S. Shopping Mall, In addition, SIPCOT Industrial Park located on OMR Pattammal Complex and Devarajan Complex. One of the fastest growing residential micro market located between Urapakkam and Potheri. ® Micro Market Overview Report | Guduvancheri, Chennai 1 Proximity to the Grand Southern Trunk (GST) Road and Outer Ring Road provides seamless connectivity. -

Government of Tamil Nadu Draft Electoral Roll Of
GOVERNMENT OF TAMIL NADU DRAFT ELECTORAL ROLL OF MUSLIM MEMBERS OF PARLIAMENT FROM TAMIL NADU 1. Thiru A. Mohammedjan - Rajya Sabha 2. Thiru K. Navaskani Lok Sabha (Ramanathapuram Constituency) (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 GOVERNMENT OF TAMIL NADU DRAFT ELECTORAL ROLL OF MUSLIM MEMBERS OF STATE LEGISLATIVE ASSEMBLY OF TAMIL NADU SI. Name of the Member Constituency No. 1. Dr. (Tmt) Nilofer Kafeel Vaniyambadi 2. Thiru M. Thamimun Ansari Nagapattinam 3. Thiru K.S. Masthan Gingee 4. Thiru T.P.M. Mohideen Khan Palayamkottai 5. Thiru K.A.M. Muhammed Abubacker Kadayanallur (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 GOVERNMENT OF TAMIL NADU DRAFT ELECTORAL ROLL OF EX- MUSLIM MEMBERS OF BAR COUNCIL OF TAMIL NADU 1. Thiru M.K. Khan 2. Thiru M. Syed Ismail (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 ELECTION OF MEMBERS OF TAMIL NADU WAQF BOARD 2020 DRAFT ELECTORAL ROLL FOR THE ELECTORAL COLLEGE OF MUTHAWALLIS OF WAQF WHOSE ANNUAL INCOME RUPEES ONE LAKH AND ABOVE 1 S. G.S. NAME OF THE WAQF NAME AND ADDRESS OF ARREARS NO. NO. INSTITUTION MUTHAWALLI / PRESIDENT / OF SECRETARY CONTRI- BUTION As on 31.03.2020 1. 2. 3. 4. 5. CHENNAI DISTRICT 1. GS.3 Ashraf Alisha & Fardalisha V.Syed Jalaludeen, Secretary, 212872 Trust, 1,St.Mary Road, 173,Kutchery Road, Mandaveli,Chennai-28 Mylapore,Chennai-4 2. -
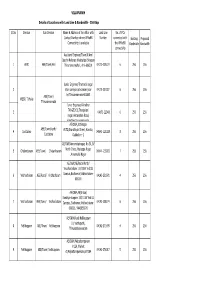
VILLUPURAM Sl.No Division Sub-Division Name & Address Of
VILLUPURAM Details of Locations with Land Line & Bandwidth - 256 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity Assistant Engineer/Town/N/Arni Opp to Nellarasi Viyabarigal Snagam 1 ARNI AEE/Town/Arni Thirumana mahal , Arni-606301 04173-225224 6 256 256 Junior Engineer/Thamarai nagar 2 Visiri samiyar ashramam(near 04175-237387 6 256 256 AEE/Town/ by)Tiruvannamalai-606603 WEST/ T.Malai Thiruvannamalai Junior Engineer/Kilnathur 3 TANGEDCO,Thenpalani 04175-223481 6 256 256 nagar,Vettavalam Road, kilnathur,Tiruvannamalai. AE/O&M,Jothinagar AEE/Town/North/ 4 Cuddalore #172,Bharathiyar Street, Kondur, 04142- 225229 8 256 256 Cuddalore Cuddalore -2 AE/O&M,Annamalainagar, No.50, IV 5 Chidambaram AEE/Town/ Chidambaram North Cross, Mariappa Nagar 04144- 239322 7 256 256 ,Annamalai Nagar AE/O&M,AE/Rural/North/ Virudhachalam 110/11KV Vrd SS 6 Vridhachalam AEE/Rural/ Vridhachalam Campus,Budhamur,Vridhachalam- 04143-231971 4 256 256 606001 AE/O&M,AE/Urban/ Kandiyankuppam 110/11KV Vrd SS 7 Vridhachalam AEE/Town/ Vridhachalam Campus, Budhamur,Vridhachalam- 04143-238274 6 256 256 606001. 9445856076 AE/O&M,Rural Nellikuppam 10, Vazhapattu, 8 Nellikuppam AEE/Town/ Nellikuppam 04142-271699 4 256 256 Thirukandeeswaram AE/O&M,Melpattampakam #12A ,Market 9 Nellikuppam AEE/Town/ Nellikuppam st,Melpattampakkam,607104 04142-276017 5 256 256 Assistant Engineer/Town I/ Villupuram, 110/11KV/SS