Ecological Co-Optation in Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract Book
Welcome to the Ornithological Congress of the Americas! Puerto Iguazú, Misiones, Argentina, from 8–11 August, 2017 Puerto Iguazú is located in the heart of the interior Atlantic Forest and is the portal to the Iguazú Falls, one of the world’s Seven Natural Wonders and a UNESCO World Heritage Site. The area surrounding Puerto Iguazú, the province of Misiones and neighboring regions of Paraguay and Brazil offers many scenic attractions and natural areas such as Iguazú National Park, and provides unique opportunities for birdwatching. Over 500 species have been recorded, including many Atlantic Forest endemics like the Blue Manakin (Chiroxiphia caudata), the emblem of our congress. This is the first meeting collaboratively organized by the Association of Field Ornithologists, Sociedade Brasileira de Ornitologia and Aves Argentinas, and promises to be an outstanding professional experience for both students and researchers. The congress will feature workshops, symposia, over 400 scientific presentations, 7 internationally renowned plenary speakers, and a celebration of 100 years of Aves Argentinas! Enjoy the book of abstracts! ORGANIZING COMMITTEE CHAIR: Valentina Ferretti, Instituto de Ecología, Genética y Evolución de Buenos Aires (IEGEBA- CONICET) and Association of Field Ornithologists (AFO) Andrés Bosso, Administración de Parques Nacionales (Ministerio de Ambiente y Desarrollo Sustentable) Reed Bowman, Archbold Biological Station and Association of Field Ornithologists (AFO) Gustavo Sebastián Cabanne, División Ornitología, Museo Argentino -

Ultimate Bolivia Tour Report 2019
Titicaca Flightless Grebe. Swimming in what exactly? Not the reed-fringed azure lake, that’s for sure (Eustace Barnes) BOLIVIA 8 – 29 SEPTEMBER / 4 OCTOBER 2019 LEADER: EUSTACE BARNES Bolivia, indeed, THE land of parrots as no other, but Cotingas as well and an astonishing variety of those much-loved subfusc and generally elusive denizens of complex uneven surfaces. Over 700 on this tour now! 1 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com Blue-throated Macaws hoping we would clear off and leave them alone (Eustace Barnes) Hopefully, now we hear of colourful endemic macaws, raucous prolific birdlife and innumerable elusive endemic denizens of verdant bromeliad festooned cloud-forests, vast expanses of rainforest, endless marshlands and Chaco woodlands, each ringing to the chorus of a diverse endemic avifauna instead of bleak, freezing landscapes occupied by impoverished unhappy peasants. 2 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com That is the flowery prose, but Bolivia IS that great destination. The tour is no longer a series of endless dusty journeys punctuated with miserable truck-stop hotels where you are presented with greasy deep-fried chicken and a sticky pile of glutinous rice every day. The roads are generally good, the hotels are either good or at least characterful (in a good way) and the food rather better than you might find in the UK. The latter perhaps not saying very much. Palkachupe Cotinga in the early morning light brooding young near Apolo (Eustace Barnes). That said, Bolivia has work to do too, as its association with that hapless loser, Che Guevara, corruption, dust and drug smuggling still leaves the country struggling to sell itself. -

Bolivia: the Andes and Chaco Lowlands
BOLIVIA: THE ANDES AND CHACO LOWLANDS TRIP REPORT OCTOBER/NOVEMBER 2017 By Eduardo Ormaeche Blue-throated Macaw www.birdingecotours.com [email protected] 2 | T R I P R E P O R T Bolivia, October/November 2017 Bolivia is probably one of the most exciting countries of South America, although one of the less-visited countries by birders due to the remoteness of some birding sites. But with a good birding itinerary and adequate ground logistics it is easy to enjoy the birding and admire the outstanding scenery of this wild country. During our 19-day itinerary we managed to record a list of 505 species, including most of the country and regional endemics expected for this tour. With a list of 22 species of parrots, this is one of the best countries in South America for Psittacidae with species like Blue-throated Macaw and Red-fronted Macaw, both Bolivian endemics. Other interesting species included the flightless Titicaca Grebe, Bolivian Blackbird, Bolivian Earthcreeper, Unicolored Thrush, Red-legged Seriema, Red-faced Guan, Dot-fronted Woodpecker, Olive-crowned Crescentchest, Black-hooded Sunbeam, Giant Hummingbird, White-eared Solitaire, Striated Antthrush, Toco Toucan, Greater Rhea, Brown Tinamou, and Cochabamba Mountain Finch, to name just a few. We started our birding holiday as soon as we arrived at the Viru Viru International Airport in Santa Cruz de la Sierra, birding the grassland habitats around the terminal. Despite the time of the day the airport grasslands provided us with an excellent introduction to Bolivian birds, including Red-winged Tinamou, White-bellied Nothura, Campo Flicker, Chopi Blackbird, Chotoy Spinetail, White Woodpecker, and even Greater Rhea, all during our first afternoon. -

BEST of WEST PAPUA 2017 Tour Report
The display of the amazing Wilson’s Bird-of-paradise was out of this world (Josh Bergmark) BEST OF WEST PAPUA 5 – 19 AUGUST 2017 LEADER: MARK VAN BEIRS and JOSH BERGMARK The incandescent Wilson’s Bird-of-paradise and the seemingly rather modestly attired Superb Bird-of- paradise were, by far, the favourite birds of our new “Best of West Papua” tour. The former because the flamboyant male showed so very well as he was cleaning his dance court and displaying a bit to his lady and the latter because we were so incredibly fortunate to be able to observe the very rarely seen full display of this fairly common and widespread, well-named species. We were the first birding tour ever to be able to offer the unique, out of this world spectacle of a dancing male Superb Bird-of-paradise to our clients! Both Birds-of-paradise were observed at close range from well positioned hides. In fact, the five most fascinating 1 BirdQuest Tour Report: Best of West Papua www.birdquest-tours.com The male Black Sicklebill on his display post (tour participant Marcel Holyoak) birds of the tour were all admired and studied from hides, as we were also lucky enough to appreciate the intricate display of a fabulous male Black Sicklebill, the wonderful ballerina dance of a male Western Parotia (for some) and the unique fashion-conscious behaviour of a decidedly unpretentiously-plumaged Vogelkop Bowerbird at his truly amazing bower. In contrast to the situation in Papua New Guinea, where hides are virtually non-existent, these simple, easily built structures make all the difference in getting the most astonishing insight in the behaviour and appreciation of some of the most appealing birds of our planet. -

Feeding Passerine and Psittacine Birds
Chapter 72 Feeding Passerine and Psittacine Birds George V. Kollias Heidi Wearne Kollias “Of all forms of life, birds are the most beautiful, most musical, most admired, most watched and most defended. Without them much of our world would seem ominously lifeless and silent.” Roger Tory Peterson toe paralysis,” which is not observed in cockatiel chicks. INTRODUCTION Cockatiels lack pigmentation (achromatosis) in their primary feathers as a result of riboflavin deficiency (Grau and One avian medicine reference states that 75% of the medical Roudybush, 1985). Although differences of this type exist ex- problems seen in companion and aviary birds have at least a perimentally, many prepared foods overcome these differences partial nutritional basis (MacWhirter, 1994). Dietary-induced by supplying levels of nutrients well in excess of the minimum deficiencies and excesses may lead to immune dysfunction, requirement for chickens. increased susceptibility to infectious diseases and metabolic and Second, and more important, many people perceive that all- biochemical derangements that manifest clinically as nutrition- seed diets (particularly diets composed of only one seed type, al secondary hyperparathyroidism, thyroid hyperplasia (dyspla- e.g., millet or sunflower) and diets composed of or are heavily sia), hemochromatosis and a variety of other problems. supplemented with, fruits, vegetables and other human foods Dietary-induced diseases frequently occur in companion and are complete foods for birds. In reality, most commercially aviary psittacine and passerine birds for several reasons. First, available seeds are deficient in certain limiting nutrients (e.g., until recently, specific nutritional requirements for these birds specific amino acids, vitamins and trace and macrominerals were unknown. -

NATURE TERRITORY April 2010 Newsletter of the Northern Territory Field Naturalists Club Inc
NATURE TERRITORY April 2010 Newsletter of the Northern Territory Field Naturalists Club Inc. PO Box 39565, Winnellie, NT 0821 President: Tida Nou 8981 6667 (h) Secretary: Ian Hance 8945 6691 (h) Treasurer: Fiona Douglas 8985 4179 (h) Membership Officer: Sherry Prince 8945 7352 (h) Journal Editors: details inside newsletter Newsletter Editor: Don Franklin 8948 1293 (h) Website Editor: Graham Brown 8945 4745 (w/h) Excursions Coordinator: Tanya Carriere 8942 0390 (h) Committee Member: Stuart Young 8995 5026 (w) Committee Member: Tissa Ratnayeke 8981 4217 (w) Club web-site: http://ntfieldnaturalists.org.au/ Meetings are generally held on the second Wednesday of every month, commencing at 7:45 PM, in Blue1.14 (Business Faculty Building) on the Casuarina Campus of Charles Darwin University. Subscriptions are on a financial-year basis and are: Families/Institutional - $30; Singles - $25; Concessions - $15. Part-year discounts are available for new members from January 1 each year. These Lycid beetles made good use of a flowering Swamp Box Lophostemon lactifluus for social contact – as did many insect species in many ways – see article and more photographs by Tissa Ratnayeke on pages 5 and 10. CONTENTS Club activities .. p2 Club notices .. p3 nest predator? .. p4 bounty .. p5 the weather .. p6-7 ecotourism .. p8-9 bird observations .. p10 recent literature .. p11 Disclaimer: The views expressed in Nature Territory are not necessarily those of the NT Field Naturalists Club Inc. or members of its Committee. Club activities April meeting. Wednesday April 14, 7:45 PM. Blue1.14 (Business Bldg.), CDU. Stephen Reynolds "Pig-nosed turtles and the Kikori region of Papua New Guinea" Steve will present a series of photos from the Kikori River drainage, which is in the Gulf of Papua region of southern Papua New Guinea. -

PEREGRINE BIRD TOURS BOLIVIA 10 – 30 November 2018 TOUR
PEREGRINE BIRD TOURS BOLIVIA 10 – 30 November 2018 TOUR REPORT LEADERS: Chris Doughty and Sandro Valdez. GROUP MEMBERS: Rebecca Albury, Graham Barwell, Paul Handreck, Elvyne Hogan, Max James and Jann Skinner. This little known and sparsely populated country, has the highest avian diversity of any land-locked country on the planet, the birding was breathtakingly exciting and produced a suite of new and interesting birds, on every day of the tour. We did particularly well with birds of prey, observing a good number of rarely seen species, which included King Vulture, Andean Condor, Swallow-tailed, Slender-billed and Double-toothed Kites, Great Black Hawk, Solitary Eagle and White-rumped and Zone-tailed Hawks. Bolivia is an outstanding destination to observe New World parrots, and we observed a total of 22 separate species of parrots, and all of them were seen particularly well. Five of them were Macaws, including two large and exceedingly rare and endemic species of Macaws, the Blue-throated and the Chestnut-fronted, which we saw splendidly well. Throughout the tour we explored a wide variety of habitats, we birded steamy Amazonian lowland rainforest, savanna grasslands, cactus-studded hillsides, elfin cloud forest and the Altiplano, high alpine plateaus, situated above the tree line, dotted with numerous small lakes. Highlights amongst the 470 species of birds we observed, were many and varied, we saw 13 of the 19 endemic birds of Bolivia and here are just some of the many highlights; Huayco Tinamou, White-bellied Nothura, the flightless Titicaca Grebe, Whistling Heron, Rufescent Tiger Heron, Plumbeous Ibis, Hoatzin, Giant Coot, Sungrebe, Red-legged Seriema, Wilson's Phalarope, Rufous-bellied Seedsnipe, Cliff, Grey-hooded and Andean Parakeets, Tucuman Amazon, Tawny-bellied Screech Owl, Andean Swift and no less than 23 species of dazzling hummingbirds. -

Animal Issues 4(2)
A N I M A L I S S U E S philosophical and ethical issues related to human/animal interactions VOLUME 4 NO. 2 2000 SYDNEY AUSTRALIA A N I M A L I S S U E S The aim of this journal is to investigate philosophical and ethical issues related to human/animal interactions. Papers are invited on any topics within this general area. Founding Editor: Dr. D. Russell, Department of General Philosophy, University of Sydney, NSW 2006, Australia e-mail: [email protected] Co-Editors: L. Birke, Institute for Women's Studies, Lancaster University, UK. B. Forsman, Centre for Research Ethics, Goteborg University, Sweden. [email protected] P. Hallen, Institute of Science and Technology Policy, Murdoch University, Western Australia. F. Mathews, School of Philosophy, La Trobe University, Victoria, Australia. V. Plumwood, Research Scholar, Research Institute for the Humanities and Social Sciences, University of Sydney. Word length should be 4,000 - 10,000 words and papers should preferably be sent on a Mac disc or by e-mail to the editor, or if this is not possible, a hard copy should be sent to the editor. Style guide available on request. Subscriptions (2000 rates): Australia and New Zealand: A$12 per issue (postage included) Back copies: A$5.00 Other countries: A$20 per issue (postage included) Back copies: A$8.00 Please see back page for payment details ANIMALISSUES Vol. 4, No. 2 2000 CONTENTS Lisa Galmark Women antivivisectionists - the story of Lizzy Lind af Hageby and Leisa Schartau 1 Simone Poirier-Bures Zaran 33 Alphonso Lingis Quadrille 37 Laura J. -
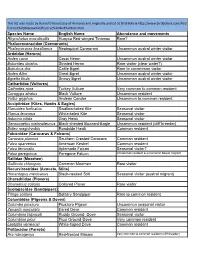
Red-Fronted Macaw Reserve Bird Checklist
This list was made by Bennett Hennessey of Armonia and originally posted at Bird Bolivia http://www.birdbolivia.com/Red‐ fronted%20Macaw%20Reserve%20Bird%20List.html Species Name English Name Abundance and movements Rhynchotus maculicollis Huayco Red-winged Tinamou Rare? Phalacrocoracidae (Cormorants) Phalacrocorax brasilianus Neotropical Cormorant Uncommon austral winter visitor Ardeidae (Herons) Ardea cocoi Cocoi Heron Uncommon austral winter visitor Butorides striatus Striated Heron Rare visitor (clear water?) Bubulcus ibis Cattle Egret Rare to uncommon visitor Ardea Alba Great Egret Uncommon austral winter visitor Egretta thula Snowy Egret Uncommon austral winter visitor Cathartidae (Vultures) Cathartes aura Turkey Vulture Very common to common resident Coragyps atratus Black Vulture Uncommon resident Vultur gryphus Andean Condor Uncommon to common resident Accipitridae (Kites, Hawks & Eagles) Elanoides forficatus Swallow-tailed Kite Seasonal visitor Elanus leucurus White-tailed Kite Seasonal visitor Asturina nitida Gray Hawk Seasonal visitor Geranoaetus melanoleucus Black-chested Buzzard-Eagle Uncommon resident (cliff breeder) Buteo magnirostris Roadside Hawk Common resident Falconidae (Caracaras & Falcons) Caracara plancus Southern Crested Caracara Common resident Falco sparverius American Kestrel Common resident Falco femoralis Aplomado Falcon Seasonal visitor? Falco peregrinus Peregrine Falcon Uncommon resident & uncommon boreal migrant Rallidae (Moorhen) Gallinula chloropus Common Moorhen Rare visitor Recurvirostridae (Avocets, -

Molecular Phylogenetics of a Clade of Lowland Tanagers: Implications for Avian Participation in the Great American Interchange
The Auk 126(3):635 648, 2009 The American Ornithologists’ Union, 2009. Printed in USA. MOLECULAR PHYLOGENETICS OF A CLADE OF LOWLAND TANAGERS: IMPLICATIONS FOR AVIAN PARTICIPATION IN THE GREAT AMERICAN INTERCHANGE KEVIN J. BURNS1 AND RACHEL A. RACICOT2 Department of Biology, San Diego State University, 5500 Campanile Drive, San Diego, California 92182, USA Abstract.—The importance of the formation of the Panamanian land bridge for mammalian diversification in the New World is well documented; however, studies investigating the role of this land bridge in avian diversification have only recently been reported. We used mitochondrial DNA data to reconstruct the phylogeny of a group of lowland tanagers (Thraupidae) that contains species distributed in both Central America and South America. Phylogenetic analyses identified a clade that includes all species in the genera Tachyphonus, Ramphocelus, Eucometis, Lanio, Trichothraupis, Coryphospingus, and Rhodospingus. Three of these species (Rhodospingus cruentus, Coryphospingus cucullatus, and C. pileatus) have traditionally been classified with the finches (Emberizidae); here, we show that they are tanagers. The genusTachyphonus is polyphyletic, with some species more closely related to species in the genus Ramphocelus than they are to other Tachyphonus. The ancestor of the entire clade was distributed in South America or was widespread there and in Central America. Reconstructing the biogeographic history of this group showed a dispersal bias from South America to Central America. Nine dispersal events were inferred, and eight of these involve dispersals from South America to Central America. Temporally, most dispersal events coincide with or postdate the final formation of the Panamanian isthmus. We also used our phylogeny to investigate plumage evolution. -

Avifaunas of the Kumawa and Fakfak Mountains, Indonesian New Guinea
Jared Diamond & K. David Bishop 292 Bull. B.O.C. 2015 135(4) Avifaunas of the Kumawa and Fakfak Mountains, Indonesian New Guinea by Jared Diamond & K. David Bishop Received 13 February 2015 Summary.—Of the 11 outlying mountain ranges along New Guinea’s north and north-west coasts, the Kumawa and Fakfak Mountains are those most isolated from the Central Range and from other outliers by flat lowlands almost at sea level. The Kumawa Mountains were previously unexplored ornithologically, and the Fakfak Mountains unexplored above 900 m. We report four surveys conducted in 1981, 1983 and 2013. The known combined avifauna is now 283 species, including 77 upland species of which the two ranges share at least 57. Among Central Range upland species whose geographic and altitudinal ranges make them plausible candidates to have colonised Fakfak and Kumawa, 15 are nevertheless unrecorded in both Fakfak and Kumawa. Of those 15, 13 are also unrecorded in the mountains of Yapen Island, which at Pleistocene times of low sea level was also separated from other New Guinea mountains by a wide expanse of flat lowlands. This suggests that colonisation of isolated mountains by those particular upland species depends on dispersal through hilly terrain, and that they do not disperse through flat lowland forest. Because of the low elevation, small area and coastal proximity of the Kumawa and Fakfak Mountains, avian altitudinal ranges there show the largest Massenerhebung effect (lowering) of any New Guinea mainland mountains known to us. We compare zoogeographic relations of the Fakfak and Kumawa avifaunas with the mountains of the Vogelkop (the nearest outlier) and with the Central Range. -

Population Status of Five Hawaiian Endemic Fern Taxa Within the Genus Diellia (Aspleniaceae)
Aguraiuja: Population status of five Hawaiian endemic fern taxa within the genus Diellia (Aspleniaceae) CBM:s Skriftserie 3: 724 Uppsala 2001 Population status of five Hawaiian endemic fern taxa within the genus Diellia (Aspleniaceae) Ruth Aguraiuja Aguraiuja, R$ 2001$ Population status of five Hawaiian endemic fern taxa within the genus Diellia (Aspleniaceae)$ CBM:s Skriftserie 3: 724$ The life-stage and size structure of 10 populations of five endemic fern taxa was studied (Diellia erecta Brack; D$ falcata Brack; D$ pallida WH Wagner; D$ unisora WH Wagner and D x lauii (D$ falcata x D$ unisora) WH Wagner), with the aim of assessing the overall condition of the populations During the study, populations were defined as spatially distinct assemblages of plants at certain sites, with no consideration of the genetic structure of the populations Spatial patchiness of habitat, clumped distribution patterns, life-stage specific survival and slow establishment of gametophytes are characteristic of all taxa of the genus Diellia Data from field observations of D$ falcata over three months lead to the assumption that Diellia ferns show a continuous growth pattern with irregular periods of dormancy and spore release with defined peaks The proportion of vegetative premature and reproductively mature sporophytes could be one possible variable for use in the assessment of the population status of Diellia ferns in short-term studies The single population of D$ pallida in Mahanaloa Valley, Kauai, is in critical condition with only 31 individu- als of