Nuclear Reactions Fission Fusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Re-Examining the Role of Nuclear Fusion in a Renewables-Based Energy Mix
Re-examining the Role of Nuclear Fusion in a Renewables-Based Energy Mix T. E. G. Nicholasa,∗, T. P. Davisb, F. Federicia, J. E. Lelandc, B. S. Patela, C. Vincentd, S. H. Warda a York Plasma Institute, Department of Physics, University of York, Heslington, York YO10 5DD, UK b Department of Materials, University of Oxford, Parks Road, Oxford, OX1 3PH c Department of Electrical Engineering and Electronics, University of Liverpool, Liverpool, L69 3GJ, UK d Centre for Advanced Instrumentation, Department of Physics, Durham University, Durham DH1 3LS, UK Abstract Fusion energy is often regarded as a long-term solution to the world's energy needs. However, even after solving the critical research challenges, engineer- ing and materials science will still impose significant constraints on the char- acteristics of a fusion power plant. Meanwhile, the global energy grid must transition to low-carbon sources by 2050 to prevent the worst effects of climate change. We review three factors affecting fusion's future trajectory: (1) the sig- nificant drop in the price of renewable energy, (2) the intermittency of renewable sources and implications for future energy grids, and (3) the recent proposition of intermediate-level nuclear waste as a product of fusion. Within the scenario assumed by our premises, we find that while there remains a clear motivation to develop fusion power plants, this motivation is likely weakened by the time they become available. We also conclude that most current fusion reactor designs do not take these factors into account and, to increase market penetration, fu- sion research should consider relaxed nuclear waste design criteria, raw material availability constraints and load-following designs with pulsed operation. -

Compilation and Evaluation of Fission Yield Nuclear Data Iaea, Vienna, 2000 Iaea-Tecdoc-1168 Issn 1011–4289
IAEA-TECDOC-1168 Compilation and evaluation of fission yield nuclear data Final report of a co-ordinated research project 1991–1996 December 2000 The originating Section of this publication in the IAEA was: Nuclear Data Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria COMPILATION AND EVALUATION OF FISSION YIELD NUCLEAR DATA IAEA, VIENNA, 2000 IAEA-TECDOC-1168 ISSN 1011–4289 © IAEA, 2000 Printed by the IAEA in Austria December 2000 FOREWORD Fission product yields are required at several stages of the nuclear fuel cycle and are therefore included in all large international data files for reactor calculations and related applications. Such files are maintained and disseminated by the Nuclear Data Section of the IAEA as a member of an international data centres network. Users of these data are from the fields of reactor design and operation, waste management and nuclear materials safeguards, all of which are essential parts of the IAEA programme. In the 1980s, the number of measured fission yields increased so drastically that the manpower available for evaluating them to meet specific user needs was insufficient. To cope with this task, it was concluded in several meetings on fission product nuclear data, some of them convened by the IAEA, that international co-operation was required, and an IAEA co-ordinated research project (CRP) was recommended. This recommendation was endorsed by the International Nuclear Data Committee, an advisory body for the nuclear data programme of the IAEA. As a consequence, the CRP on the Compilation and Evaluation of Fission Yield Nuclear Data was initiated in 1991, after its scope, objectives and tasks had been defined by a preparatory meeting. -

Uranium (Nuclear)
Uranium (Nuclear) Uranium at a Glance, 2016 Classification: Major Uses: What Is Uranium? nonrenewable electricity Uranium is a naturally occurring radioactive element, that is very hard U.S. Energy Consumption: U.S. Energy Production: and heavy and is classified as a metal. It is also one of the few elements 8.427 Q 8.427 Q that is easily fissioned. It is the fuel used by nuclear power plants. 8.65% 10.01% Uranium was formed when the Earth was created and is found in rocks all over the world. Rocks that contain a lot of uranium are called uranium Lighter Atom Splits Element ore, or pitch-blende. Uranium, although abundant, is a nonrenewable energy source. Neutron Uranium Three isotopes of uranium are found in nature, uranium-234, 235 + Energy FISSION Neutron uranium-235, and uranium-238. These numbers refer to the number of Neutron neutrons and protons in each atom. Uranium-235 is the form commonly Lighter used for energy production because, unlike the other isotopes, the Element nucleus splits easily when bombarded by a neutron. During fission, the uranium-235 atom absorbs a bombarding neutron, causing its nucleus to split apart into two atoms of lighter mass. The first nuclear power plant came online in Shippingport, PA in 1957. At the same time, the fission reaction releases thermal and radiant Since then, the industry has experienced dramatic shifts in fortune. energy, as well as releasing more neutrons. The newly released neutrons Through the mid 1960s, government and industry experimented with go on to bombard other uranium atoms, and the process repeats itself demonstration and small commercial plants. -

Radioactive Decay
North Berwick High School Department of Physics Higher Physics Unit 2 Particles and Waves Section 3 Fission and Fusion Section 3 Fission and Fusion Note Making Make a dictionary with the meanings of any new words. Einstein and nuclear energy 1. Write down Einstein’s famous equation along with units. 2. Explain the importance of this equation and its relevance to nuclear power. A basic model of the atom 1. Copy the components of the atom diagram and state the meanings of A and Z. 2. Copy the table on page 5 and state the difference between elements and isotopes. Radioactive decay 1. Explain what is meant by radioactive decay and copy the summary table for the three types of nuclear radiation. 2. Describe an alpha particle, including the reason for its short range and copy the panel showing Plutonium decay. 3. Describe a beta particle, including its range and copy the panel showing Tritium decay. 4. Describe a gamma ray, including its range. Fission: spontaneous decay and nuclear bombardment 1. Describe the differences between the two methods of decay and copy the equation on page 10. Nuclear fission and E = mc2 1. Explain what is meant by the terms ‘mass difference’ and ‘chain reaction’. 2. Copy the example showing the energy released during a fission reaction. 3. Briefly describe controlled fission in a nuclear reactor. Nuclear fusion: energy of the future? 1. Explain why nuclear fusion might be a preferred source of energy in the future. 2. Describe some of the difficulties associated with maintaining a controlled fusion reaction. -

An Introduction to the IAEA Neutron-Gamma Atlas Ye Myint1, Sein Htoon2
MM0900107 Jour. Myan. Aca<L Arts & Sa 2005 Vol. in. No. 3(i) Physics An Introduction to the IAEA Neutron-Gamma Atlas Ye Myint1, Sein Htoon2 Abstract NG-ATLAS is the abbreviation of Neutron-Gamma Atlas. Neutrons have proved to be especially effective in producing nuclear transformations, and gamma is the informer of nucleus. In nuclear physics, observable features or data are determined externally such as, energy, spin and cross section, where neutron and gamma plays dominant roles. (Neutron, gamma) (n,y) reactions of every isotope are vital importance for nuclear physicists. Introduction Inside the nucleus, protons and neutrons are simply two different states of a single particle, the nucleon. When neutron leaves the.nucleus, it is an unstable particle of half life 11.8 minutes, beta decay into proton. It is not feasible to describe beta decay without the references to Dirac 's hole theory; it is the memorandum for his hundred-birth day . The role of neutrons in nuclear physics is so high and its radiative capture by elements (neutron, gamma) reaction i.e, (n,y ) or NG-ATLAS is presented. The NG-Atlas. data file contains nuclear reaction cross section data of induced capture reaction for targets up to Z =96 . It comprises more than seven hundred isotopes, if the half lives are above half of a day. Cross sections are listed separately for ground , first and second excited states .And if the isomers have half life longer than 0.5 days , they are also include as targets .So the information includes a total of about one thousand reactions on different channels and the incident neutron energy ranging fromlO"5 eV to 20 MeV. -

Nuclear Reactors Fuelled with Uranium Inevitably Produce Plutonium As a By-Product
The Secretary Joint Standing Committee on Treaties Inquiry into Nuclear Non-proliferation and Disarmament Dear Sir/Madam, I would like to submit the following submission to the Parliamentary Committee Inquiry into Nuclear Non- proliferation and Disarmament. Yours sincerely, Frank Barnaby. A submission to the Parliamentary Committee Inquiry into Nuclear Non- proliferation and Disarmament. Frank Barnaby Nuclear reactors fuelled with uranium inevitably produce plutonium as a by-product. This plutonium can be used by countries and by nuclear terrorists to fabricate nuclear weapons. The operation of nuclear-power reactors, therefore, has consequences for national, regional and global security. The more nuclear reactors there are the greater the security risks. Australia should recognise that these security risks outweigh the befits of producing electricity by nuclear power especially because the use of renewable sources of energy, combined with improvements in energy efficiency and the conservation of energy make the use of nuclear power unnecessary. As the world’s second largest exporter of uranium, Australia has a major responsibility to adopt policies to minimise the risks to security from nuclear proliferation and terrorism. To this end, Australia should use its influence to bring the Comprehensive Nuclear Test Ban Treaty (CTBT) into effect. It should not supply uranium to countries, like the USA and China, which have not yet ratified the CTBT. Moreover, Australia should promote the negotiation of a Comprehensive Fissile Material Cut-Off Treaty to prohibit the further production of fissile material usable for the production of nuclear weapons, prohibit the reprocessing of spent nuclear-power reactor fuel that has been produced by Australian uranium and should not support or encourage the use of Mixed Oxide (MOX) nuclear fuel or the use of Generation IV reactors, particularly fast breeder reactors. -

Highly Enriched Uranium: Striking a Balance
OFFICIAL USE ONLY - DRAFT GLOSSARY OF TERMS APPENDIX F GLOSSARY OF TERMS Accountability: That part of the safeguards and security program that encompasses the measurement and inventory verification systems, records, and reports to account for nuclear materials. Assay: Measurement that establishes the total quantity of the isotope of an element and the total quantity of that element. Atom: The basic component of all matter. Atoms are the smallest part of an element that have all of the chemical properties of that element. Atoms consist of a nucleus of protons and neutrons surrounded by electrons. Atomic energy: All forms of energy released in the course of nuclear fission or nuclear transformation. Atomic weapon: Any device utilizing atomic energy, exclusive of the means for transportation or propelling the device (where such means is a separable and divisible part of the device), the principal purpose of which is for use as, or for development of, a weapon, a weapon prototype, or a weapon test device. Blending: The intentional mixing of two different assays of the same material in order to achieve a desired third assay. Book inventory: The quantity of nuclear material present at a given time as reflected by accounting records. Burnup: A measure of consumption of fissionable material in reactor fuel. Burnup can be expressed as (a) the percentage of fissionable atoms that have undergone fission or capture, or (b) the amount of energy produced per unit weight of fuel in the reactor. Chain reaction: A self-sustaining series of nuclear fission reactions. Neutrons produced by fission cause more fission. Chain reactions are essential to the functioning of nuclear reactors and weapons. -

Fundamentals of Nuclear Power
Fundamentals of Nuclear Power Juan S. Giraldo Douglas J. Gotham David G. Nderitu Paul V. Preckel Darla J. Mize State Utility Forecasting Group December 2012 Table of Contents List of Figures .................................................................................................................................. iii List of Tables ................................................................................................................................... iv Acronyms and Abbreviations ........................................................................................................... v Glossary ........................................................................................................................................... vi Foreword ........................................................................................................................................ vii 1. Overview ............................................................................................................................. 1 1.1 Current state of nuclear power generation in the U.S. ......................................... 1 1.2 Nuclear power around the world ........................................................................... 4 2. Nuclear Energy .................................................................................................................... 9 2.1 How nuclear power plants generate electricity ..................................................... 9 2.2 Radioactive decay ................................................................................................. -

Nuclear Chemistry Why? Nuclear Chemistry Is the Subdiscipline of Chemistry That Is Concerned with Changes in the Nucleus of Elements
Nuclear Chemistry Why? Nuclear chemistry is the subdiscipline of chemistry that is concerned with changes in the nucleus of elements. These changes are the source of radioactivity and nuclear power. Since radioactivity is associated with nuclear power generation, the concomitant disposal of radioactive waste, and some medical procedures, everyone should have a fundamental understanding of radioactivity and nuclear transformations in order to evaluate and discuss these issues intelligently and objectively. Learning Objectives λ Identify how the concentration of radioactive material changes with time. λ Determine nuclear binding energies and the amount of energy released in a nuclear reaction. Success Criteria λ Determine the amount of radioactive material remaining after some period of time. λ Correctly use the relationship between energy and mass to calculate nuclear binding energies and the energy released in nuclear reactions. Resources Chemistry Matter and Change pp. 804-834 Chemistry the Central Science p 831-859 Prerequisites atoms and isotopes New Concepts nuclide, nucleon, radioactivity, α− β− γ−radiation, nuclear reaction equation, daughter nucleus, electron capture, positron, fission, fusion, rate of decay, decay constant, half-life, carbon-14 dating, nuclear binding energy Radioactivity Nucleons two subatomic particles that reside in the nucleus known as protons and neutrons Isotopes Differ in number of neutrons only. They are distinguished by their mass numbers. 233 92U Is Uranium with an atomic mass of 233 and atomic number of 92. The number of neutrons is found by subtraction of the two numbers nuclide applies to a nucleus with a specified number of protons and neutrons. Nuclei that are radioactive are radionuclides and the atoms containing these nuclei are radioisotopes. -

X-Ray and Neutron Sources
Chapter 1: Radioactivity Radioactivity from Direct Excitation X‐ray Emission 147 NPRE 441, Principles of Radiation Protection An Overview of Radiation Exposure to US Population 148 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity Clinical X‐ray CT System The Siemens SOMATOM X clinical CT system 149 Chapter 1: Radioactivity X‐ray Imaging Examples 150 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity X‐ray Generation –X‐ray Tube Andrew Webb, Introduction to Biomedical Imaging, 2003, Wiley‐ Interscience. Motor, Why? Filament Rotating target Electron beam? How are electrons generated? 151 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity X‐ray Generation – Bremsstrahlung • Target nucleus positive charge (Z∙p+) attracts incident e‐ • Deceleration of an incident e‐ occurs in the proximity of the target atom nucleus • Energylostbye‐ is gained by the EM photon (x‐ray) generated • The impact parameter distance, the closest approach to the nucleus by the e‐ determines the amount of E loss • The Coulomb force of attraction varies strongly with distance ( 1/r2); ↓ distance →↑deceleration and E loss →↑photon E • Direct impact on the nucleus determines the maximum x‐ray E (Emax) 152 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity X‐ray Generation – Bremsstrahlung Interestingly, this process creates a relatively uniform spectrum. Intensity = nh 0 Photon energy spectrum 153 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity The Unfiltered Bremsstrahlung Spectrum Thick Target X‐ray Formation We can model target as a series of thin targets. Electrons successively loses energy as they moves deeper into the target. Electrons X‐rays Relative Intensity 0 Each layer produces a flat energy spectrum with decreasing peak energy level. -

Chapter 14 NUCLEAR FUSION
Chapter 14 NUCLEAR FUSION For the longer term, the National Energy Strategy looks to fusion energy as an important source of electricity-generating capacity. The Department of Energy will continue to pursue safe and environmentally sound approaches to fusion energy, pursuing both the magnetic confinement and the inertial confinement concepts for the foreseeable future. International collaboration will become an even more important element of the magnetic fusion energy program and will be incorporated into the inertial fusion energy program to the fullest practical extent. (National Energy Strategy, Executive Summary, 1991/1992) Research into fundamentally new, advanced energy sources such as [...] fusion energy can have substantial future payoffs... [T]he Nation's fusion program has made steady progress and last year set a record of producing 10.7 megawatts of power output at a test reactor supported by the Department of Energy. This development has significantly enhanced the prospects for demonstrating the scientific feasibility of fusion power, moving us one step closer to making this energy source available sometime in the next century. (Sustainable Energy Strategy, 1995) 258 CHAPTER 14 Nuclear fusion is essentially the antithesis of the fission process. Light nuclei are combined in order to release excess binding energy and they form a heavier nucleus. Fusion reactions are responsible for the energy of the sun. They have also been used on earth for uncontrolled release of large quantities of energy in the thermonuclear or ‘hydrogen’ bombs. However, at the present time, peaceful commercial applications of fusion reactions do not exist. The enormous potential and the problems associated with controlled use of this essentially nondepletable energy source are discussed briefly in this chapter. -
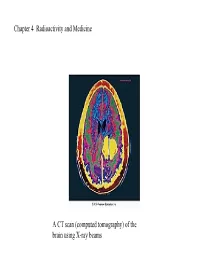
Chapter 4 Radioactivity and Medicine a CT Scan (Computed Tomography)
Chapter 4 Radioactivity and Medicine A CT scan (computed tomography) of the brain using X-ray beams A radioactive isotope has an unstable nucleus; it emits radiation to become more stable and can be one or more of the isotopes of an element Alpha (α) particle +2 is two protons and two neutrons, a helium nucleus Beta (β) particle is a high-energy electron Positron (β+) is a positive electron (an extract from a proton) Gamma (γ ) ray is high-energy released from a nucleus in the form of electromagnetic radiation Radiation protection requires paper and clothing for alpha particles a lab coat or gloves for beta particles a lead shield or a thick concrete wall for gamma rays limiting the amount of time spent near a radioactive source increasing your distance from the source Note that the atomic mass and atomic number are reversed in the equation but that the sum of the atomic masses and numbers in the reactant (U) equals the sum in the products (Th and He) Remember that the mass of an electron is very small compared to neutrons and protons and that a neutron can be thought of as a proton and electron combined 19 Write an equation for the decay of K42 (potassium-42), a beta (electron) emitter. 19 -1 K42 eo + ? Mass number of ? same (42) Atomic number of ? This electron is emitted from the nucleus, not from one of the outer electrons A neutron must have been split to give an electron and a proton 0 + -1 n1 = p1 + e0 20 ?42 20 Ca42 +1 In positron emission, a proton is converted to a neutron and positron, e0 49 +1 Mn25 e0 + ? In this case the nucleus