Amlodipine 5Mg and 10Mg Tablets Amlodipine Mesilate Monohydrate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potentially Harmful Drugs in the Elderly: Beers List
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− March 2019 ~ Resource #350301 Potentially Harmful Drugs in the Elderly: Beers List In 1991, Dr. Mark Beers and colleagues published a methods paper describing the development of a consensus list of medicines considered to be inappropriate for long-term care facility residents.12 The “Beers list” is now in its sixth permutation.1 It is intended for use by clinicians in outpatient as well as inpatient settings (but not hospice or palliative care) to improve the care of patients 65 years of age and older.1 It includes medications that should generally be avoided in all elderly, used with caution, or used with caution or avoided in certain elderly.1 There is also a list of potentially harmful drug-drug interactions in seniors, as well as a list of medications that may need to be avoided or have their dosage reduced based on renal function.1 This information is not comprehensive; medications and interactions were chosen for inclusion based on potential harm in relation to benefit in the elderly, and availability of alternatives with a more favorable risk/benefit ratio.1 The criteria no longer address drugs to avoid in patients with seizures or insomnia because these concerns are not unique to the elderly.1 Another notable deletion is H2 blockers as a concern in dementia; evidence of cognitive impairment is weak, and long-term PPIs pose risks.1 Glimepiride has been added as a drug to avoid. Some drugs have been added with cautions (dextromethorphan/quinidine, trimethoprim/sulfamethoxazole), and some have had cautions added (rivaroxaban, tramadol, SNRIs). -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

LIST of APPROVED DRUG from 01-01-2011 to 31-12-2011
LIST OF APPROVED DRUG FROM 01-01-2011 to 31-12-2011 Date of Sr. No. Name of Drug Indication Issue Alpha Lipoic Acid USP 100mg + Methylcobalamin 1500mcg + Vitamin B6 IP 3mg + Folic Acid IP 1.5mg + Benfotiamine For the treatment of diabetic 1. 03.01.11 50mg + Biotin USP 5mg + Chromium neuropathy Picolinate USP Eq. to Chromuim 200mcg Capsule Ropinirole ER Tablet 1 mg. 2. Same as approved 04.01.11 (Additional Strength) Monotherapy for the Maintenance Treatment of Patients with locally advanced metastatic Erlotinib HCl Tablet 150 mg 3. non-small lung cancer whose disease has not 04.01.11 (Additional Indication) progressed after four cycles of Platinum based First Line Chemotherapy. Moxifloxacin HCl BP 0.5% w/v + Bromfenac For the reduction of post operative 4. 05.01.11 Sodium 0.09% w/v Eye drop inflammatory conditions of the eye Ferrous Ascorbate 100mg + Folic Acid IP 1.5mg + Cyanocobalamin IP 15mcg + Zinc 5. For the treatment of iron deficiency anaemia 05.01.11 Sulphate Monohydrate Eq. to Elemental Zinc 22.5mg Tablet S (+) Etodolac 300mg +Thiocolchicoside 8mg For the treatment of patients with acute 6. 05.01.11 Tablet painful musculoskeletal conditions Beclomethasone Dipropionate IP 100mcg + For the treatment of bronchial asthma where Formoterol Fumarate Dihydrate BP Eq. to 7. use of inhaled corticosteroid therapy found 05.01.11 Formoterol Fumarate 6 mcg Metered Dose appropriate Inhaler Amlodipine Besilate IP Eq. to Amlodipine For the treatment of mild to moderate 8. 05.01.11 5mg + Indapamide USP SR 1.5mg Tablet hypertension Metformin HCl IP 500mg + Alpha Lipoic Acid For the treatment of patients with diabetic 9. -

Calcium Channel Blockers
Calcium Channel Blockers Summary In general, calcium channel blockers (CCBs) are used most often for the management of hypertension and angina. There are 2 classes of CCBs: the dihydropyridines (DHPs), which have greater selectivity for vascular smooth muscle cells than for cardiac myocytes, and the non-DHPs, which have greater selectivity for cardiac myocytes and are used for cardiac arrhythmias. The DHPs cause peripheral edema, headaches, and postural hypotension most commonly, all of which are due to the peripheral vasodilatory effects of the drugs in this class of CCBs. The non-DHPs are negative inotropes and chronotropes; they can cause bradycardia and depress AV node conduction, increasing the risk of heart failure exacerbation, bradycardia, and AV block. Clevidipine is a DHP calcium channel blocker administered via continuous IV infusion and used for rapid blood pressure reductions. All CCBs are substrates of CYP3A4, but both diltiazem and verapamil are also inhibitors of 3A4 and have an increased risk of drug interactions. Verapamil also inhibits CYP2C9, CYP2C19, and CYP1A2. Pharmacology CCBs selectively inhibit the voltage-gated L-type calcium channels on cardiac myocytes, vascular smooth muscle cells, and cells within the sinoatrial (SA) and atrioventricular (AV) nodes, preventing influx of extracellular calcium. CCBs act by either deforming the channels, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the major cellular calcium store, the endoplasmic reticulum. Calcium influx via these channels serves for excitation-contraction coupling and electrical discharge in the heart and vasculature. A decrease in intracellular calcium will result in inhibition of the contractile process of the myocardial smooth muscle cells, resulting in dilation of the coronary and peripheral arterial vasculature. -
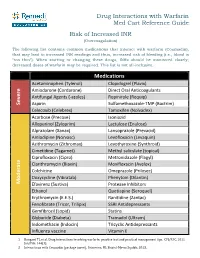
Drug Interactions with Warfarin Med Cart Reference Guide Risk of Increased INR (Overcoagulation)
Drug Interactions with Warfarin Med Cart Reference Guide Risk of Increased INR (Overcoagulation) The following list contains common medications that interact with warfarin (Coumadin), that may lead to increased INR readings and thus, increased risk of bleeding (i.e., blood is “too thin”). When starting or changing these drugs, INRs should be monitored closely; decreased doses of warfarin may be required. This list is not all-inclusive. Medications Acetaminophen (Tylenol) Clopidogrel (Plavix) Amiodarone (Cordarone) Direct Oral Anticoagulants Antifungal Agents (‐azoles) Ropinirole (Requip) Severe Aspirin Sulfamethoxazole‐TMP (Bactrim) Celecoxib (Celebrex) Tamoxifen (Nolvadex) Acarbose (Precose) Isoniazid Allopurinol (Zyloprim) Lactulose (Enulose) Alprazolam (Xanax) Lansoprazole (Prevacid) Amlodipine (Norvasc) Levofloxacin (Levaquin) Azithromycin (Zithromax) Levothyroxine (Synthroid) Cimetidine (Tagamet) Methyl salicylate (topical) Ciprofloxacin (Cipro) Metronidazole (Flagyl) Clarithromycin (Biaxin) Moxifloxacin (Avelox) Colchicine Omeprazole (Prilosec) Doxycycline (Vibratab) Phenytoin (Dilantin) Moderate Efavirenz (Sustiva) Protease Inhibitors Ethanol Quetiapine (Seroquel) Erythromycin (E.E.S.) Ranitidine (Zantac) Fenofibrate (Tricor, Trilipix) SSRI Antidepressants Gemfibrozil (Lopid) Statins Glyburide (Diabeta) Tramadol (Ultram) Indomethacin (Indocin) Tricyclic Antidepressants Influenza vaccine Vitamin E 1. Bungard TJ, et al. Drug interactions involving warfarin: practice tool and practical management tips. CPJ/RPC. 2011 Jan/Feb. 144(1). 2. Interactions with Coumadin [package insert]. Princeton, NJ: Bristol‐Myers Squibb; 2013. Drug Interactions with Warfarin Med Cart Reference Guide Risk of Decreased INR (Undercoagulation) The following list contains common medications that interact with warfarin (Coumadin), which may lead to decreased INR readings and thus, increased risk of clotting, strokes, and DVTs (i.e., blood is “not thin enough”). When starting or changing these drugs, INRs should be monitored closely; increased doses of warfarin may be required. -

Oral Calcium Channel Blocker Comparison
Oral Calcium Channel Blocker Comparison Various calcium channel blockers (CCBs) have been periodically shorted. Below is a table of dosing comparisons. General notes: No dose equivalencies among the CCBs have been established; estimate an approximate dose using the dosing ranges. The contraindications and adverse effects of non-dihydropyridine (DHP) CCBs (diltiazem and verapamil) are quite different from DHP CCBs (amlodipine, felodipine, nifedipine). Consider staying with the same type of CCB if possible unless other considerations warrant changing types. Be sure to check for drug interactions if switching agents. Calcium Channel Blocker Comparisons1,2 Doses CCB Contraindications Hypertension Stable angina DHP Adverse Effects: pedal edema, flushing, palpitations, headache Nifedipine MR 30-60 mg up to 90 mg daily 2.5-5 mg to 10 mg Amlodipine 5-10 mg daily daily severe aortic stenosis 2.5-10 mg to May be useful but Felodipine 20 mg daily not indicated Non-DHP Adverse Effects: angina, heart failure; constipation, especially with verapamil 120-240 mg post myocardial infarction with 120-180 mg to 360 Diltiazem MR to 360 mg ejection fraction (EF) <40% mg daily daily 2nd or 3rd degree AV block, or sick sinus syndrome (unless functioning ventricular pacemaker) atrial flutter/atrial fibrillation and accessory bypass tract (e.g. Wolff- 80-240 mg Parkinson-White syndrome, Lown- 180 mg to 480 mg once daily to Ganong-Levine syndrome) Verapamil MR daily in one or two 180-240 mg combination with ivabradine doses BID Verapamil extreme bradycardia severe heart failure and or EF<40% combination with drugs that affect cardiac conduction CCB= calcium channel blocker; DHP= dihydropyridine; MR=modified release such as XL, CD, SR, etc. -

Effects of a New Calcium Channel Blocker, Azelnidipine, on Systemic Hemodynamics and Renal Sympathetic Nerve Activity in Spontaneously Hypertensive Rats
1017 Hypertens Res Vol.28 (2005) No.12 p.1017-1023 Original Article Effects of a New Calcium Channel Blocker, Azelnidipine, on Systemic Hemodynamics and Renal Sympathetic Nerve Activity in Spontaneously Hypertensive Rats Takatomi SHOKOJI*, Yoshihide FUJISAWA**, Hideyasu KIYOMOTO***, Matlubur RAHMAN*,***, Guang-Ping SUN***, Yu-Yan FAN*, Shoji KIMURA*, Masakazu KOHNO***, Youichi ABE*, and Akira NISHIYAMA* Antihypertensive treatment with dihydropyridine calcium channel blockers elicits sympathetic nerve activa- tion, which may contribute to cardiovascular events. However, recent clinical studies showed that treatment with azelnidipine, a new dihydropyridine calcium channel blocker, significantly reduced blood pressure in hypertensive patients while either maintaining or actually decreasing heart rate (HR). In this study, we exam- ined the effects of azelnidipine and amlodipine on systemic hemodynamics and renal sympathetic nerve activity (RSNA) in anesthetized spontaneously hypertensive rats (SHR). We also examined the effects of these agents on baroreflex functions by infusing phenylephrine (30 µg/kg/min, i.v.) and sodium nitroprus- side (10 µg/kg/min, i.v.) into azelnidipine- or amlodipine-treated SHR. Fifty min after administration of azelni- dipine (10 µg/kg/min for 10 min, i.v.), mean arterial pressure (MAP) significantly decreased from 153±5 to 122±5 mmHg; however, HR and integrated RSNA did not change significantly (from 352±9 to 353±10 beats/ min and 115±5% of baseline, respectively). Infusion of amlodipine (50 µg/kg/min for 10 min) elicited similar effects on MAP (from 152±5 to 120±4 mmHg). However, amlodipine significantly increased HR (from 351±9 to 375±11 beats/min) and integrated RSNA (165±5% of baseline). -

Phamacokinetics of Phenytoin: Unaltered by Enalapril and Amlodipine in Rhesus Monkeys
Indian J Physiol Pharmacol 1999; 43 (2): 251-254 PHAMACOKINETICS OF PHENYTOIN: UNALTERED BY ENALAPRIL AND AMLODIPINE IN RHESUS MONKEYS D. K. BADYAL, S. K. GARG*, V. K. BHARGAVA AND S. MAJUMDARt Departments of Pharmacology and Experimental Medicine t, Post Graduate Institute of Medical Education and Research, Chandigarh - 160 012 ( Received on September 28, 1998 ) Abstract: A cross over single and multiple dose study was carried out to find out pharmacokinetic interactions between diphynylhydantoin (DPH) (35 mglkg, po) and antihypertensives enalapril (1.6 mg/kg; po) and amlodipine (0.4 mg/kg, po) in rhesus monkeys. Neither the plasma concentrations nor the pharmacokinetic parameters of DPH were altered by coadministration of enalapril or amlodipine, suggesting that enalapril and amlodipine can be safely administered to epileptic patients receiving phenytoin. Key words: phenytoin amlodipine interaction enalapril rhesus monkey INTRODUCTION plasma concentration ranges between 10-20 mg/L (7). Hypertension and epilepsy are generally independent disorders but may sometimes Interactions between antihypertensives co-exist in the same patient (1, 2). Both and anticonvulsants may lead to increase ailments require long term treatment. in their adverse effects or loss of control over the disorder. Hence, the present study Enalapril, an angiotensin converting was designed to evaluate the effect of enzyme (ACE) inhibitor is converted to its enalapril and amlodipine on the pharmac active moiety in the liver (3). Amlodipine is okinetics of phenytoin in -

List of Formulary Drug Removals
July 2021 Formulary Drug Removals Below is a list of medicines by drug class that have been removed from your plan’s formulary. If you continue using one of the drugs listed below and identified as a Formulary Drug Removal, you may be required to pay the full cost. If you are currently using one of the formulary drug removals, ask your doctor to choose one of the generic or brand formulary options listed below. Category Formulary Drug Formulary Options Drug Class Removals Acromegaly SANDOSTATIN LAR SOMATULINE DEPOT SIGNIFOR LAR SOMAVERT Allergies dexchlorpheniramine levocetirizine Antihistamines Diphen Elixir RyClora CARBINOXAMINE TABLET 6 MG Allergies BECONASE AQ flunisolide spray, fluticasone spray, mometasone spray, DYMISTA Nasal Steroids / Combinations OMNARIS QNASL ZETONNA Anticonvulsants topiramate ext-rel capsule carbamazepine, carbamazepine ext-rel, clobazam, divalproex sodium, (generics for QUDEXY XR only) divalproex sodium ext-rel, gabapentin, lamotrigine, lamotrigine ext-rel, levetiracetam, levetiracetam ext-rel, oxcarbazepine, phenobarbital, phenytoin, phenytoin sodium extended, primidone, rufinamide, tiagabine, topiramate, valproic acid, zonisamide, FYCOMPA, OXTELLAR XR, TROKENDI XR, VIMPAT, XCOPRI BANZEL SUSPENSION clobazam, lamotrigine, rufinamide, topiramate, TROKENDI XR ONFI SABRIL vigabatrin ZONEGRAN carbamazepine, carbamazepine ext-rel, divalproex sodium, divalproex sodium ext-rel, gabapentin, lamotrigine, lamotrigine ext-rel, levetiracetam, levetiracetam ext-rel, oxcarbazepine, phenobarbital, phenytoin, phenytoin sodium -

Adverse Drug Reactions Study of Antihypertensive Drugs in Primary Care Settings
JMPF Vol. 10 No. 4 : 241-248 ISSN-p : 2088-8139 ISSN-e : 2443-2946 Adverse Drug Reactions Study of Antihypertensive Drugs in Primary Care Settings Yeni Farida*, Kharimah Faizathus Tsalatsatun Universitas Sebelas Maret, Surakarta, Jawa Tengah Submitted: 17-06-2020 Revised: 24-09-2020 Accepted: 18-12-2020 Korespondensi : Yeni Farida : Email : [email protected] ABSTRACT Hypertension is one of the high-prevalence diseases in primary care. Failure to achieve the target of blood pressure is affected by non-compliance due to the antihypertensive adverse reactions. This study aims to determine adverse drug reaction (ADR) of antihypertensive drugs in primary care settings. A cross sectional study was conducted in “Sibela” Primary Care in Surakarta on March 2019. Investigators interviewed patients directly and observed supporting data from medical records. Hypertension patients with antihypertensive drugs at least for a month were eligible in this study. Then, the data were analyzed by the Liverpool algorithm that interpreted in 4 scales: unlikely, possible, probable, and definite. A total 70 subject were dominated by female (80%). Monotherapy of antihypertensive drugs prescribed to patient in primary care were amlodipine (80%) and captopril (10%). Nine events of ADR were found in hypertension patient. None ADR were doubtful. Possible ADR of amlodipine was drowsiness (5.4%), whereas probable ADR were nausea (3.4%), diuresis (1.8%), and abdominal pain (1.8 %). Definite ADR of captopril was dry mouth (14.3%) and probable ADR was abdominal pain (14.3%). Further investigation regarding the drowsiness, ADR of amlodipine, was needed. Keywords: antihypertensive drugs; Liverpool Algorithm; primary care; ADR INTRODUCTION Study about ADR in hypertension Based on the health profile of Central treatment was important to be conducted for Java province, and especially Surakarta city in optimizing patient safety. -

Table 1: Drug-Drug Interactions of Common Cardiac Drugs and Chemotherapeutic Agents*
Table 1: Drug-Drug Interactions of Common Cardiac Drugs and Chemotherapeutic Agents* Cardiac Drug(s) Enzyme/ Chemotherapy Effect of Drug- Suggested Oncologist Suggested Cardiologist Action Drug† Drug Management Management Interaction Beta-Blockers All beta- Additive Ceritinib Additive Avoid using the combination of ceritinib with beta- blockers clinical bradycardia blockers. If concomitant use is necessary and symptomatic effect bradycardia occurs, hold ceritinib, adjust or discontinue the beta-blocker, and upon recovery resume ceritinib at a reduced dose with frequent monitoring of heart rate.‡ Crizotinib Monitor blood pressure and heart rate regularly. Dose reduction or discontinuation of one of the agents may be necessary if clinically significant bradycardia occurs.‡ Carvedilol P-gp Afatinib ↑ Monitor for adverse Consider alternative agent if inhibition chemotherapy effects of afatinib. If possible. (moderate) drug not well-tolerated, concentration decrease afatinib daily dose by 10 mg. Doxorubicin Monitor for adverse Consider alternative agent if Nilotinib effects of possible. If carvedilol is used for Paclitaxel chemotherapy drug if prevention of anthracycline Pazopanib concomitant therapy is cardiotoxicity, individual risk vs. Vincristine necessary. benefit must be considered. If Vinblastine concomitant therapy is necessary and drug-drug interaction involves QT- prolonging chemotherapy drug, ensure appropriate electrocardiographic (ECG) and electrolyte monitoring. Carvedilol; CYP2D6 Imatinib ↑ beta-blocker Monitor blood pressure -

Brand Name: Aptiom Generic Name: Eslicarbazepine Manufacturer: Sunovion Pharmaceuticals Inc1 Product Availability: FDA Approved November 2013
Brand Name: Aptiom Generic Name: eslicarbazepine Manufacturer: Sunovion Pharmaceuticals Inc1 Product Availability: FDA approved November 2013. Anticipated availability is late 2014.2 Drug Class: Anticonvulsant, central nervous system agent, voltage-gated sodium channel blocker 3,4 Uses: Labeled uses: Adjunctive treatment of partial seizures3,4 Unlabeled uses: None o Under investigation for use as monotherapy for treatment of focal epilepsy or partial-onset seizures5 o Under investigation as a possible treatment for bipolar disorder3,5,6 Mechanism of Action: Like many other anticonvulsants, the exact mechanism of action of eslicarbazepine is unknown. The antiepileptic action of the drug is theorized to be due to the blockage of voltage-gated sodium channels. Studies indicate that eslicarbazepine and its metabolites competitively interact with voltage-gated sodium channels to inhibit sustained repetitive neuronal firing, with enhanced inhibitory selectivity for rapidly firing neurons.1,3 Pharmacokinetics: Absorption: 1,3 Tmax 1-4 hours Vd 61 L t ½ 13-20 hours Clearance 20 mL/min GFR 80-120 mL/min Protein binding < 40% (% to albumin unknown) Bioavailability > 90% Metabolism: 1,3 Eslicarbazepine is rapidly metabolized to its major active metabolite eslicarbazepine (91%) by hydrolytic first-pass metabolism. Minor active metabolites are R-licarbazepine (5%) and oxcarbazepine (1%). In in vitro studies, eslicarbazepine acetate was found to be a moderate inhibitor of CYP2C19 and an inducer of CYP3A4. Studies with eslicarbazepine in fresh human hepatocytes showed only mild activation of UGT1A1- mediated glucuronidation. Eslicarbazepine showed no clinical inhibitory effects on CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1, and CYP3A4 and did not undergo autoinduction. Elimination: 1,3 Eslicarbazepine metabolites are eliminated primarily by renal excretion in the unchanged and glucuronide conjugate forms.