Aluminum As a Structural Material
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigating the Durability of Structures by Dana Saba Bachelor of Engineering, Mcgill University, Montréal, 2011
Investigating the Durability of Structures by Dana Saba Bachelor of Engineering, McGill University, Montréal, 2011 Submitted to the Department of Civil and Environmental Engineering in Partial Fulfillment of the Requirements for the Degree of Master of Engineering in Civil and Environmental Engineering at the Massachusetts Institute of Technology June 2013 © 2013 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Civil and Environmental Engineering May 10th, 2013 Certified by: Jerome J. Connor Professor of Civil and Environmental Engineering Thesis Supervisor Certified by: Rory Clune Massachusetts Institute of Technology Thesis Reader Accepted by: Heidi Nepf Chair, Departmental Committee for Graduate Students 2 Investigating the Durability of Structures by Dana Saba Submitted to the Department of Civil and Environmental Engineering in May 10, 2013, in Partial Fulfillment of the Requirements for the Degree of Master of Engineering in Civil and Environmental Engineering Abstract The durability of structures is one of primary concerns in the engineering industry. Poor durability in design may result in a structure losing its performance to the extent where structural integrity is no longer satisfied and human lives are at stake. Moreover, the associated costs of maintenance and repair due to inadequate design considerations are high. Thus, designing for durable structures not only helps sustain our infrastructure, it also reduces future costs. This thesis identifies the key factors that define and impact durability, with particular attention paid to the effect of material choice on overall durability. This follows a study of the different deteriorating mechanisms that wood, steel and reinforced concrete undergo over time, and the different enhancement techniques used to reduce the adverse effects of these mechanisms. -

Material Quantities in Building Structures and Their Environmental Impact
Material quantities in building structures and their environmental impact by Catherine De Wolf B.Sc., M.Sc. in Civil Architectural Engineering Vrije Universiteit Brussel and Université Libre de Bruxelles, 2012 Submitted to the Department of Architecture in Partial Fulfillment of the Requirements for the Degree of Master of Science in Building Technology at the Massachusetts Institute of Technology June 2014 © 2014 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Architecture May 9, 2014 Certified by: John A. Ochsendorf Professor of Architecture and Civil and Environmental Engineering Thesis Supervisor Accepted by: Takehiko Nagakura Associate Professor of Design and Computation Chair of the Department Committee on Graduate Students John E. Fernández Professor of Architecture, Building Technology, and Engineering Systems Head, Building Technology Program Co-director, International Design Center, MIT Thesis Reader Frances Yang Structures and Sustainability Specialist at Arup Thesis Reader “It is […] important to remember that unlike operational carbon emissions the embodied carbon cannot be reversed” Craig Jones, Circular Ecology Material quantities in building structures and their environmental impact by Catherine De Wolf Submitted to the Department of Architecture in Partial Fulfillment of the Requirements for the Degree of Master of Science in Building Technology on May 9, 2014. Thesis Supervisor: John Ochsendorf Title Supervisor: Professor of Architecture and Civil and Environmental Engineering -

Applications of Aluminium Alloys in Civil Engineering
T. Dokšanović i dr. Primjene aluminijskih legura u građevinarstvu ISSN 1330-3651 (Print), ISSN 1848-6339 (Online) https://doi.org/10.17559/TV-20151213105944 APPLICATIONS OF ALUMINIUM ALLOYS IN CIVIL ENGINEERING Tihomir Dokšanović, Ivica Džeba, Damir Markulak Subject review Although aluminium is a long known structural material, its use is not in accordance with the benefits achieved by its implementation. There are several reasons for such an adverse state. Those that stand out are the late development of a regulative framework for the design of structures, and still the need for improvement, lack of knowledge on application examples and not stressed enough potential areas of use. However, positive trends are present and aluminium alloys are competitive, especially if their positive properties can be utilized and negative properties diminished through purpose oriented design approach. Given examples present good application utilization of aluminium alloys characteristics, and shown new application research can provide directions for further expansion of competitiveness. Structural uses are grouped in suitable areas of application and put into local and global context. Keywords: bridge; façade; refurbishment; roof system; seismic Primjene aluminijskih legura u građevinarstvu Pregledni članak Iako je aluminij već dugo prisutan konstrukcijski materijal, uporaba mu nije u skladu s dobrobitima koji se ostvaruju njegovom primjenom. Nekoliko je uzročnika takvog stanja. Ističu se relativno kasni razvoj normativnog okvira za dimenzioniranje konstrukcija, i još uvijek potreba za poboljšanjem, nedovoljna raširenost saznanja o primjenama te nedostatno naglašena potencijalna područja primjene. Međutim, pozitivni trendovi su prisutni, a legure aluminija su konkurentne, posebno ako se njegova pozitivna svojstva mogu iskoristiti, a negativna umanjiti kroz proračunski pristup orijentiran prema namjeni. -

Designing for Durability CONTINUING EDUCATION Strategies for Achieving Maximum Durability with Wood-Frame Construction Sponsored by Rethink Wood
EDUCATIONAL-ADVERTISEMENT Designing for Durability EDUCATION CONTINUING Strategies for achieving maximum durability with wood-frame construction Sponsored by reThink Wood rchitects specify wood for many Examples of wood buildings that have (glulam), cross laminated timber (CLT), and reasons, including cost, ease and stood for centuries exist all over the world, nail-laminated timber, along with a variety A efficiency of construction, design including the Horyu-ji temple in Ikaruga, of structural composite lumber products, are versatility, and sustainability—as well as Japan, built in the eighth century, stave enabling increased dimensional stability and its beauty and the innate appeal of nature churches in Norway, including one in Urnes strength, and greater long-span capabilities. and natural materials. Innovative new built in 1150, and many more. Today, wood These innovations are leading to taller, technologies and building systems are also is being used in a wider range of buildings highly innovative wood buildings. Examples leading to the increased use of wood as a than would have been possible even 20 years include (among others) a 10-story CLT structural material, not only in houses, ago. Next-generation lumber and mass timber apartment building in Australia, a 14-story schools, and other traditional applications, products, such as glue-laminated timber timber-frame apartment in Norway, but in larger, taller, and more visionary wood buildings. But even as the use of wood is expanding, one significant characteristic of wood buildings is often underestimated: their durability. Misperceptions still exist that buildings made of materials such as concrete or steel last longer than buildings made of wood. -

Structural Composite Materials Tailored for Damping
L Journal of Alloys and Compounds 355 (2003) 216–223 www.elsevier.com/locate/jallcom S tructural composite materials tailored for damping D.D.L. Chung* Composite Materials Research Laboratory, University at Buffalo, State University of New York, Buffalo, NY 14260-4400, USA Abstract This paper reviews the tailoring of structural composite materials for damping. By the use of the interfaces and viscoelasticity provided by appropriate components in a composite material, the damping capacity can be increased with negligible decrease, if any, of the stiffness. In the case of cement–matrix composites, the use of silica fume as an admixture results in increases in both the damping capacity and the storage modulus. In the case of continuous fiber polymer–matrix lightweight composites, the use of submicron-diameter discontinuous carbon filaments as an interlaminar additive is more effective than the use of an interlaminar viscoelastic layer in enhancing the loss modulus when the temperature exceeds 50 8C. Surface treatment of the composite components is important. In the case of steel reinforced concrete, the steel reinforcing bar (rebar) contributes much to the damping, but appropriate surface treatment of the rebar further enhances the damping. 2003 Elsevier Science B.V. All rights reserved. Keywords: Disordered systems; Polymers; Elastomers and plastics; Surfaces and interfaces; Elasticity; Anharmonicity 1 . Introduction [2–4] is the goal of the structural material tailoring described in this review paper. The development of materials for vibration and acoustic Vibrations are undesirable for structures, due to the need damping has been focused on metals and polymers [1]. for structural stability, position control, durability (par- Most of these materials are functional materials rather than ticularly durability against fatigue), performance, and noise practical structural materials due to their high cost, low reduction. -

Structural Materials
OPTI 222 Mechanical Design in Optical Engineering Structural Materials Beryllium Among the light metals, beryllium is characterized by its out-standing combination of properties--low density (0.067 lb/in3), high strength (60 psi), good thermal properties, low cross-section, to thermal neutrons and high melting point (2300 °F). The use of beryllium has nevertheless been restricted by its poor ductility and low resistance to impact, as well as by factors of its toxicity and high cost. Some of the properties of beryllium are summarized as follows: 1. Density: Beryllium "has a relatively low density of 0.067 lbs/in3. This is slightly higher than the density of magnesium and about two-thirds that of aluminum. 2. Modulus of Elasticity: The modulus of elasticity of Beryllium high, being 3.1 x 104 kg/mm2 (44 x 106 psi). This combined with its low density, makes it attractive as a light, but stiff, material. 3. Tensile Properties: The mechanical properties of beryllium are affected by the method of production. Hot-pressed beryllium has a room temperature ultimate tensile strength of around 60 Ksi with a ductility of 0.5 - 0.3%. Beryllium retains its strength at high temperatures; even at 600 °C, it has an ultimate tensile strength of about 25 Ksi. Above 250 °C, its ductility rises to a level in excess of 10%. The fatigue strength of beryllium is high, being in excess of 80% of the ultimate tensile strength. Its room temperature impact strength, however, is negligible and in this respect it performs more like a ceramic than a metal. -

Material Quantities in Building Structures and Their Environmental Impact
Material quantities in building structures and their environmental impact by Catherine De Wolf B.Sc., M.Sc. in Civil Architectural Engineering Vrije Universiteit Brussel and Université Libre de Bruxelles, 2012 Submitted to the Department of Architecture in Partial Fulfillment of the Requirements for the Degree of Master of Science in Building Technology at the Massachusetts Institute of Technology June 2014 © 2014 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Architecture May 9, 2014 Certified by: John A. Ochsendorf Professor of Architecture and Civil and Environmental Engineering Thesis Supervisor Accepted by: Takehiko Nagakura Associate Professor of Design and Computation Chair of the Department Committee on Graduate Students 2 John E. Fernández Professor of Architecture, Building Technology, and Engineering Systems Head, Building Technology Program Co-director, International Design Center, MIT Thesis Reader Frances Yang Structures and Sustainability Specialist at Arup Thesis Reader 3 “It is […] important to remember that unlike operational carbon emissions the embodied carbon cannot be reversed” Craig Jones, Circular Ecology 4 Material quantities in building structures and their environmental impact by Catherine De Wolf Submitted to the Department of Architecture in Partial Fulfillment of the Requirements for the Degree of Master of Science in Building Technology on May 9, 2014. Thesis Supervisor: John Ochsendorf Title Supervisor: Professor of Architecture and Civil and Environmental Engineering Abstract Improved operational energy efficiency has increased the percentage of embodied energy in the total life cycle of building structures. Despite a growing interest in this field, practitioners lack a comprehensive survey of material quantities and embodied carbon in building structures. -

Alternative Reinforcement Approaches
Alternative Reinforcement Approaches Extended service life of exposed concrete structures Master of Science Thesis in the Master’s Programme Structural Engineering and Building Technology JOHAN KARLSSON Department of Civil and Environmental Engineering Division of Structural Engineering Concrete Structures CHALMERS UNIVERSITY OF TECHNOLOGY Göteborg, Sweden 2014 Master’s Thesis 2014:151 MASTER’S THESIS 2014:151 Alternative Reinforcement Approaches Extended service life of exposed concrete structures Master of Science Thesis in the Master’s Programme Structural Engineering and Building Technology JOHAN KARLSSON Department of Civil and Environmental Engineering Division of Structural Engineering CHALMERS UNIVERSITY OF TECHNOLOGY Gothenburg, Sweden 2014 Alternative Reinforcement Approaches Extended service life of concrete structures Master of Science Thesis in the Master’s Programme Structural Engineering and Building Technology JOHAN KARLSSON © JOHAN KARLSSON, 2014 Examensarbete/Institutionen för bygg- och miljöteknik, Chalmers tekniska högskola 2014:151 Department of Civil and Environmental Engineering Division of Structural Engineering Chalmers University of Technology SE-412 96 Gothenburg Sweden Telephone: + 46 (0)31-772 1000 Cover: Rusted reinforcement bars (Gudmundsson 2008). Department of Civil and Environmental Engineering, Gothenburg, Sweden 2014 Alternative Reinforcement Approaches Extended service life of concrete structures Master of Science Thesis in the Master’s Programme Structural Engineering and Building Technology JOHAN KARLSSON Department of Civil and Environmental Engineering Division of Structural Engineering Chalmers University of Technology ABSTRACT Concrete is the most used construction material today, the vast majority of it reinforced with ordinary reinforcing steel. Much research has been put into improving the protection of steel reinforcement by increasing concrete cover and impenetrability. However, even though concrete quality has indeed improved much in the last decades, reinforcement corrosion is still a serious problem. -

Influence of Loading Speed on Tensile Strength Characteristics of High Tensile Steel
Influence of loading speed on tensile strength characteristics of high tensile steel S.M.YANG, H.Y.KANG, H.S.KIM, J.H.SONG, J.M.PARK Department of Mechanical Engineering, Chonbuk National University, Chonju, 561-756, R.O. Korea ABSTRACT The purpose of this paper is presented a rational method of predicting dynamic/impact tensile strength of high tensile steel materials widely used for structural material of automobiles. It is known that the ultimate strength is related with the loading speed and the Lethargy Coefficient from the tensile test. The Lethargy Coefficient is proportional to the disorientation of the molecular structure and indicates the magnitude of defects resulting from the probability of breaking the bonds responsible for its strength. The coefficient is obtained from the simple tensile test such as failure time and stresses at fracture. These factors not only affect the static strength but also have a great influence on the dynamic/impact characteristics of the joint and the adjacent structures. This strength is used to analyze the failure life prediction of mechanical system by virtue of its material fracture. The impact tensile test is performed to evaluate the life parameters due to loading speed with the proposed method. Also the evaluation of the dynamic/impact effect on the material tensile strength characteristics is compared with the result of Campbell-Cooper equation to verify the proposed method. NOMENCLATURE W : Life ( sec ) W 0 : Life Coefficient (sec ) U 0 : Bonding Energy ( KJ / mole ) k : Boltzmann Constant -
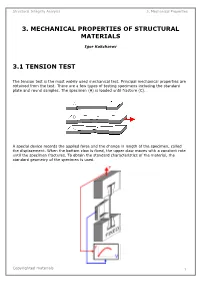
Structural Integrity Analysis 3
Structural Integrity Analysis 3. Mechanical Properties 3. MECHANICAL PROPERTIES OF STRUCTURAL MATERIALS Igor Kokcharov 3.1 TENSION TEST The tension test is the most widely used mechanical test. Principal mechanical properties are obtained from the test. There are a few types of testing specimens including the standard plate and round samples. The specimen (A) is loaded until fracture (C). A special device records the applied force and the change in length of the specimen, called the displacement. When the bottom claw is fixed, the upper claw moves with a constant rate until the specimen fractures. To obtain the standard characteristics of the material, the standard geometry of the specimen is used. Copyrighted materials 1 Structural Integrity Analysis 3. Mechanical Properties There are round and plate specimens. They have reinforced ends with smooth transitions to the middle. The middle part has a constant cross-sectional area which can be round (A) or rectangular (B). Structural materials have different relationships between the applied force and displacement of the specimen. Curve A is typical for rigid and high-strength materials such as tool alloys or boron fibers. Curve B is typical for carbon and alloyed steels. Curve C is typical for aluminum and other nonferrous alloys. Curve D is typical for nonmetallic materials such as plastic or rubber. An applied external force F stretches the specimen. There is an average elongation and a local elongation in the specimen. Strain is a measure of the elongation. The tensile strain is defined as the change per unit length due to a force. At the final stage before fracture, plastic deformation concentrates in a section. -

Civil Engineering Materials SAB 2112 Introduction to Steel
10/9/2010 Civil Engineering Materials SAB 2112 Introduction to Steel Dr Mohamad Syazli Fathi Department of Civil Engineering RAZAK School of Engineering & Advanced Technology UTM International Campus October 9, 2010 CONTENT SCHEDULE – 5th Meeting 1. Types and application of steel in construction 2. Non-ferrous metal - types and characteristics, use of non-ferrous metal in construction 3. Latest construction materials - polymer, glass, composite material, cement based products 1 10/9/2010 Learning Objectives 1. Understand different types of structural steels used in construction. 2. Discuss about the stress-strain relationship of structural steel. 3. Highlight the advantages and disadvantages of using steel as structural material. 4. Discuss fatigue failure and it’s significance. 5. Brittle fracture of steel. 6. Fire performance and protection of the steel. 7. Corrosion of steel and it’s protection. Introduction: Steel • Steels are essentially alloys of iron and carbon but they always contain other elements, either as impurities or alloying elements. • Steel is man made metal containing 95% or more iron and 1 – 2% carbon, smaller amounts (around 1.6%) of manganese, nickel to improve certain properties. • Carbon improves strength/hardness but reduces ductility and toughness. • Low carbon steels are not used as structural materials. • Alloying nickel, the tensile strength can be increased while retaining the desired ductility. 2 10/9/2010 Types of Steel Steel •Low carbon steel (mild steel) •Medium carbon steel •High carbon steel (tool steels) •Cast iron Alloy Steels •Stainless steel •High speed steel 5 Low Carbon Steel Also known as mild steel Contain 0.05% -0.32% carbon 1. -

Review of Structural Material Corrosion in Liquid Li and Pb-Li
Review of Structural Material Corrosion in Liquid Li and Pb-Li S. Sharafat and N. M. Ghoniem The University of California at Los Angeles (UCLA) Los Angeles, CA. 90095-1597, USA APEX Meeting Sandia National Laboratory November 15-17, 2000 SS-NG: Nov.'00 APEX-SNL 1 OUTLINE • Liquid metal corrosion examples • Review in conjunction with Na-Steel Data effects of: ∆ – Velocity, Tmax, T, Impurities – Product deposition rates – Rate of Temperature Rise – Coatings • Systems: – Steel-Lithium – V-Lithium – Steel Pb-Li –V-Pb-Li •Summary SS-NG: Nov.'00 APEX-SNL 2 Examples of Liquid Metal Corrosion MOLTEN LEAD: T = 700oC ∆T = 150oC V = 3 cm/s ALLOYS: • Hasetlloy: – 70% Ni; – 8000 ppm at 700oC •Croloy: N –2LQCr, 1Mo, 0.5Mn; – 8 ppm at 700oC • Fecralloy: – 15Cr, 4Al • Meehanite: – cast Fe, 3C, 1Si R. C. Asher et al., 1977 SS-NG: Nov.'00 APEX-SNL 3 Example of Lithium-Steel Corrosion • Formation of a ferritic layer on 316 SS: – Leaching of Cr, Ni, and Fe • Nitrogen solubility in Li is high even at Tm (~1000 ppm): – Nitrogen must be removed for iron-based heat transfer systems – For Vanadium-based systems Nitrogen inhibits certain corrosions • Austenitic 316 Stainless Steel in Li: o Tmax = 425 C R. C. Asher et al., 1977 N = 200 -500 ppm t > 3000 hr SS-NG: Nov.'00 APEX-SNL 4 Corrosion Damage Zones • Corrosion is often described in terms of a simple mass loss rate: – Mass loss rate (g/m2-year), or – Surface recession rate (µm/year) Total Damaged Zone • However, 3 processes constitute corrosion: – Surface Regression (wall thinning) J.H.DeVan, 1985 – Surface