Summary of Federal Emergency Stimulus Bills March 27 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Dance Division Calendar 17-18
Early Dance Division 2017-2018 Session 1 September 9 – November 3 Monday Classes Tuesday Classes September 11 Class September 12 Class September 18 Class September 19 Class September 25 Class September 26 Class October 2 Class October 3 Class October 9 Class October 10 Class October 16 Class October 17 Class October 23 Class October 24 Class October 30 Last Class October 31 Last Class Wednesday Classes Thursday Classes September 13 Class September 14 Class September 20 Class September 21* Class September 27 Class September 28 Class October 4 Class October 5 Class October 11 Class October 12 Class October 18 Class October 19 Class October 25 Class October 26 Class November 1 Last Class November 2 Last Class Saturday Classes Sunday Classes September 9 Class September 10 Class September 16 Class September 17 Class September 23 Class September 24 Class September 30* Class October 1 Class October 7 Class October 8 Class October 14 Class October 15 Class October 21 Class October 22 Class October 28 Last Class October 29 Last Class *Absences due to the holiday will be granted an additional make-up class. Early Dance Division 2017-2018 Session 2 November 4 – January 22 Monday Classes Tuesday Classes November 6 Class November 7 Class November 13 Class November 14 Class November 20 No Class November 21 No Class November 27 Class November 28 Class December 4 Class December 5 Class December 11 Class December 12 Class December 18 Class December 19 Class December 25 No Class December 26 No Class January 1 No Class January 2 No Class January 8 Class -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Coronavirus (COVID-19) Situational Update for March 4, 2021
Thursday, March 4, 2021 WHERE WE ARE TODAY 2 March 4, 2021 SAFE AND EFFECTIVE VACCINES 3 The United States now has three safe and effective COVID-19 vaccines: Moderna (2-dose) All the available vaccines have been Pfizer (2-dose) proven effective at preventing serious Janssen / Johnson & Johnson (1-dose) illness, hospitalization, and death from Washingtonians are encouraged to get the first COVID-19. vaccine available to you. March 4, 2021 VACCINATION APPOINTMENTS 4 This is the final week that we will use the current system to book appointments, and DC Health and the Office of the Chief Technology Officer (OCTO) have been working with Microsoft to ensure a better user experience. On Thursday, March 4 at 9:00 a.m., 5,750 appointments opened to: § DC residents who live in priority zip codes and are 65 and older § DC residents who live in priority zip codes and are 18-64 with a qualifying medical condition On Friday, March 5 at 9:00 a.m., 5,750 appointments will open to: § DC residents 65 and older § DC residents 18-64 with a qualifying medical condition March 4, 2021 IMPROVEMENTS TO VACCINATE.DC.GOV 5 Improvements to vaccinate.dc.gov include: increased server availability for the portal the addition of a “waiting room” that will only allow 3,000 users to access the appointment questionnaire at a time to improve the user experience and increase accessibility, the CAPTCHA has been removed from the vaccinate.dc.gov questionnaire March 4, 2021 A NEW PATH FORWARD FOR VACCINATION APPOINTMENTS 6 Beginning next week, the District will begin using a pre-registration system for making vaccination appointments. -
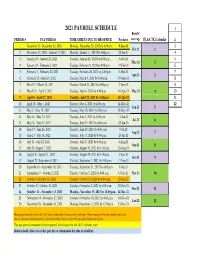
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -
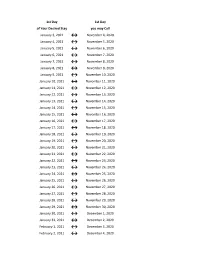
Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
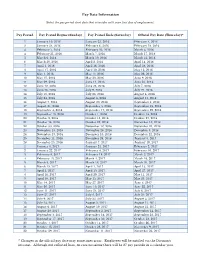
Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

COVID-19 in Ontario: Focus on March 21, 2021 to March 27, 2021
Weekly Epidemiologic Summary COVID-19 in Ontario: Focus on March 21, 2021 to March 27, 2021 This report includes the most current information available from CCM as of March 30, 2021. Please visit the interactive Ontario COVID-19 Data Tool to explore recent COVID-19 data by public health unit, age group, sex, and trends over time. A daily summary is available and provides an epidemiologic summary of recent COVID-19 activity in Ontario. This weekly report provides an epidemiologic summary of COVID-19 activity in Ontario over time. Highlights There are a total of 344,436 confirmed cases of COVID-19 in Ontario with a public health unit reported date up to March 27, 2021. For the period with a public health unit reported date between March 21 to 27, 2021 (week 12): A total of 14,360 cases were reported to public health compared to 11,014 cases the previous week (March 14 to 20, 2021). There is a 30.4% increase in reported cases in Ontario this week (n=14,360) compared to the previous week (n=11,014). The last time the week-to-week percentage increase was this large was the week of December 29th, 2020. In week 12, the most ethnically diverse neighbourhoods in Ontario had COVID-19 rates that were 3.8 times higher than the least diverse neighbourhoods. This is the largest rate ratio reported since week 8 (February 21 to 27, 2021). The term public health unit reported date in this document refers to the date local public health units were first notified of the case. -

One Hundred Sixteenth Congress of the United States of America
H. R. 748 One Hundred Sixteenth Congress of the United States of America AT THE SECOND SESSION Begun and held at the City of Washington on Friday, the third day of January, two thousand and twenty An Act To amend the Internal Revenue Code of 1986 to repeal the excise tax on high cost employer-sponsored health coverage. Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled, SECTION 1. SHORT TITLE. This Act may be cited as the ‘‘Coronavirus Aid, Relief, and Economic Security Act’’ or the ‘‘CARES Act’’. SEC. 2. TABLE OF CONTENTS. The table of contents for this Act is as follows: Sec. 1. Short title. Sec. 2. Table of contents. Sec. 3. References. DIVISION A—KEEPING WORKERS PAID AND EMPLOYED, HEALTH CARE SYSTEM ENHANCEMENTS, AND ECONOMIC STABILIZATION TITLE I—KEEPING AMERICAN WORKERS PAID AND EMPLOYED ACT Sec. 1101. Definitions. Sec. 1102. Paycheck protection program. Sec. 1103. Entrepreneurial development. Sec. 1104. State trade expansion program. Sec. 1105. Waiver of matching funds requirement under the women’s business cen- ter program. Sec. 1106. Loan forgiveness. Sec. 1107. Direct appropriations. Sec. 1108. Minority business development agency. Sec. 1109. United States Treasury Program Management Authority. Sec. 1110. Emergency EIDL grants. Sec. 1111. Resources and services in languages other than English. Sec. 1112. Subsidy for certain loan payments. Sec. 1113. Bankruptcy. Sec. 1114. Emergency rulemaking authority. TITLE II—ASSISTANCE FOR AMERICAN WORKERS, FAMILIES, AND BUSINESSES Subtitle A—Unemployment Insurance Provisions Sec. 2101. Short title. Sec. 2102. Pandemic Unemployment Assistance. Sec. 2103. Emergency unemployment relief for governmental entities and nonprofit organizations. -

Tracking the Covid-19 Economy Weekly Labor Market Information
TRACKING THE COVID-19 ECONOMY WEEKLY LABOR MARKET INFORMATION Week ending March 20, 2021 Unemployment Insurance (UI) Claims for the Three-State Region For the week ending March 20, 2021, workers filed 30,028 initial UI claims (NSA) in our three-state region — fewer than the prior week’s 32,124 (revised), and far below the historic peak of 599,446 for the week ending March 28, 2020. Workers also filed 18,898 initial Pandemic Unemployment Assistance (PUA) claims for the week ending March 20, 2021, in our three states. The PUA program provides benefits for workers who are not eligible for regular UI benefits. Initial UI claims plus initial PUA claims were 48,926 for the week ending March 20. Prior to the pandemic, the 52- week average of initial UI claims was about 24,000 for the week ending March 14, 2020. Compared with the prior week, initial UI claims across our three states: . fell to 1,392 from 1,914 (revised) in Delaware; . fell to 9,404 from 10,272 (revised) in New Jersey; and . fell to 19,232 from 19,938 (revised) in Pennsylvania. Millions, NSA Millions, NSA 0.7 6 0.6 5 Initial PUA 0.5 4 0.4 Continued PUA 3 0.3 PEUC 2 0.2 0.1 1 0.0 0 3/7 4/18 5/30 7/11 8/22 10/3 11/14 12/26 2/6 3/20 3/7 4/18 5/30 7/11 8/22 10/3 11/14 12/26 2/6 3/20 2020 2021 2020 2021 Source: Bureau of Labor Statistics via Haver Analytics; not seasonally adjusted (NSA) For the week ending March 13, 2021, continued UI claims in our three-state region fell to 406,287 (NSA) — from the prior week’s 425,927 (revised). -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November -

ELECTION DAY EARLY VOTING March
EAST BATON ROUGE SAMPLE BALLOT U.S. Representative 2nd Congressional District ❍ Chelsea Ardoin-- Republican, Female, White ition for Equity & Justice ❍ Belden ‘Noonie Man’ Batiste-- Independent, Male, Black Powered by the Power Coal ❍ Claston Bernard-- Republican, Male, Black ❍ Troy A. Carter-- Democrat, Male, Black EARLY VOTING ❍ Karen Carter Peterson-- Democrat, Female, Black March 6-13 ❍ Gary Chambers-- Democrat, Male, Black ❍ Harold John-- Democrat, Male, Black Polls Open 8:30 AM - 6:00 PM CST (Except Sunday, March 7) ❍ J. Christopher Johnson-- Democrat, Male, Black ❍ Brandon Jolicoeur-- No Party, Male, White ELECTION DAY ❍ Lloyd M. Kelly-- Democrat, Male, Black March 20, 2021 ❍ ‘Greg’ Lirette-- Republican, Male, White Polls are open 7:00 AM - 8:00 PM CST ❍ Mindy McConnell-- Libertarian, Female, White ❍ Desiree Ontiveros-- Democrat, Female, Other Vote by Mail Vote by Mail PowerCoalition.org/Vote ❍ Jenette M. Porter-- Democrat, Female, Black ❍ Sheldon C. Vincent-- Republican, Male, Black If you are planning to vote by mail, we recommend you do so as soon as possible to make sure your vote is counted. Council Member District 1, City of Central REQUEST RETURN a Vote by Mail a Completed Council members are tasked with representing the interests of their Ballot: Vote by Mail Ballot: constituents. In addition to proposing, passing, and ratifying laws and ordinances, city councils manage budgets and investigate city Mar. 16 Mar. 19 agencies when necessary. ❍ Tim Lazaroe-- Republican, Male, White Louisiana has added five more reasons ❍ Wayne Messina-- Republican, Male, White for people to request an absentee ballot during the COVID-19 pandemic. Find out if you are eligible: PowerCoalition.org/Vote Unopposed Races--Automatically Elected Having Problems Voting? Member of School Board District 6, Zachary Community: Report a voting incident or Elecia Brown ‘Lisa’ Lathon-- Democrat, Female, Black get assistance from trained volunteers by calling 1-866-OUR-VOTE Your VOICE matters.