Of Escherichia Coli 0157:H7+
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fermented and Ripened Fish Products in the Northern European Countries
Accepted Manuscript Fermented and ripened fish products in the Northern European countries Torstein Skåra, Lars Axelsson, Gudmundur Stefánsson, Bo Ekstrand, Helge Hagen PII: S2352-6181(15)00005-0 DOI: 10.1016/j.jef.2015.02.004 Reference: JEF 12 To appear in: Journal of Ethnic Foods Received Date: 16 January 2015 Revised Date: 23 January 2015 Accepted Date: 2 February 2015 Please cite this article as: Skåra T, Axelsson L, Stefánsson G, Ekstrand B, Hagen H, Fermented and ripened fish products in the Northern European countries, Journal of Ethnic Foods (2015), doi: 10.1016/ j.jef.2015.02.004. This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT 1 Fermented and ripened fish products in the Northern European countries 2 Torstein Skåra 1* , Lars Axelsson 2, Gudmundur Stefánsson 3, Bo Ekstrand 4 and Helge Hagen 5 3 1 Nofima - Norwegian Institute of Food, Fisheries, and Aquaculture Research, Postboks 8034, 4 NO-4068 Stavanger, Norway 5 2 Nofima - Norwegian Institute of Food, Fisheries, and Aquaculture Research, P.O.Box 210, 6 NO-1431 Ås, Norway 7 3 Matis, Vinlandsleid 12, 113 Reykjavik, Iceland 8 4 Bioconsult AB, Stora Vägen 49, SE-523 61 Gällstad, Sweden 5 MANUSCRIPT 9 Dælivegen 118, NO-2385 Brumunddal, Norway 10 *Author for correspondence: Tel: +47-51844600; Fax: +47-51844651 11 E-mail. -

Open Mckinney Thesis 4 1.Pdf
The Pennsylvania State University The Graduate School College of Agricultural Sciences INVESTIGATION OF FOOD SAFETY PARAMETERS FOR FERMENTED SEMI-DRY AND DRY SAUSAGE PRODUCTS A Thesis in Animal Science by Samantha R. McKinney 2017 Samantha R. McKinney Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science May 2017 The thesis of Samantha R. McKinney was reviewed and approved* by the following: Jonathan A. Campbell Assistant Professor of Animal Science Extension Meat Specialist Thesis Advisor Catherine N. Cutter Professor of Food Science Food Safety Extension Specialist – Muscle Foods Nancy M. Ostiguy Associate Professor of Entomology Terry D. Etherton Distinguished Professor of Animal Nutrition Head of the Department of Animal Science *Signatures are on file in the Graduate School ii ABSTRACT Fermentation and drying are two methods utilized by humans for thousands of years to preserve food. Fermented semi-dry and dry sausages are safe, ready-to-eat (RTE) meat items produced using strict government regulations. One of these regulations requires meat processing establishments to create and have a scientifically-validated Hazard Analysis Critical Control Point (HACCP) plan. HACCP plans are validated utilizing a combination of data collected in the plant and scientific literature to ensure that process controls exist for identified food safety hazards. When little or incomplete data exists for very specific products or processes, challenge studies may be conducted to investigate the safety of the processes used to produce the food item. Three experiments were conducted to determine the effects of varying fermented semi-dry and dry sausage production parameters on the reduction of three pathogenic bacteria: E. -

The Evaluation of Pathogen Survival in Dry Cured Charcuterie Style Sausages
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2019 THE EVALUATION OF PATHOGEN SURVIVAL IN DRY CURED CHARCUTERIE STYLE SAUSAGES Jennifer Michelle McNeil University of Kentucky, [email protected] Digital Object Identifier: https://doi.org/10.13023/etd.2019.074 Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation McNeil, Jennifer Michelle, "THE EVALUATION OF PATHOGEN SURVIVAL IN DRY CURED CHARCUTERIE STYLE SAUSAGES" (2019). Theses and Dissertations--Animal and Food Sciences. 102. https://uknowledge.uky.edu/animalsci_etds/102 This Master's Thesis is brought to you for free and open access by the Animal and Food Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Animal and Food Sciences by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

Dry-Cured Meat Products According to the Smoking Regime: Process Optimization to Control Polycyclic Aromatic Hydrocarbons
foods Article Dry-Cured Meat Products According to the Smoking Regime: Process Optimization to Control Polycyclic Aromatic Hydrocarbons Maria João Fraqueza 1 , Marta Laranjo 2 , Susana Alves 1 , Maria Helena Fernandes 1, Ana Cristina Agulheiro-Santos 2,3 , Maria José Fernandes 1, Maria Eduarda Potes 2,4 and Miguel Elias 2,3,* 1 CIISA-Centro de Investigação Interdisciplinar em Sanidade Animal, Faculdade de Medicina Veterinária, Universidade de Lisboa, Avenida da Universidade Técnica, 1300-477 Lisboa, Portugal; [email protected] (M.J.F.); [email protected] (S.A.); [email protected] (M.H.F.); [email protected] (M.J.F.) 2 MED-Mediterranean Institute for Agriculture, Environment and Development, IIFA-Instituto de Investigação e Formação Avançada, Universidade de Évora, Pólo da Mitra, Ap. 94, 7006-554 Évora, Portugal; [email protected] (M.L.); [email protected] (A.C.A.-S.); [email protected] (M.E.P.) 3 Departamento de Fitotecnia, Escola de Ciências e Tecnologia, Universidade de Évora, Pólo da Mitra, Ap. 94, 7006-554 Évora, Portugal 4 Departamento de Medicina Veterinária, Escola de Ciências e Tecnologia, Universidade de Évora, Pólo da Mitra, Ap. 94, 7006-554 Évora, Portugal * Correspondence: [email protected] Received: 11 December 2019; Accepted: 11 January 2020; Published: 15 January 2020 Abstract: The manufacturing of dry-cured meat products usually includes a smoking step. Polycyclic aromatic hydrocarbons (PAHs) are potentially carcinogenic chemical compounds that may result from smoking. The aim of the present study was to optimize the smoking regime of traditional dry-cured meat products in order to minimize the presence of PAHs. -

Non-Alcoholic Fatty Liver Disease
NON-ALCOHOLIC FATTY LIVER DISEASE — A GUIDE TO — WHAT& HOW TO E AT TABLE OF CONTENTS PART 1 PART 2 • THE NORMAL LIVER • NUTRITION BASICS • THE LIVER WITH NON-ALCOHOLIC • WHAT ARE THE DIFFERENT FATTY LIVER DISEASE (NAFLD) FOOD GROUPS? • STAGES OF NON-ALCOHOLIC FATTY LIVER DISEASE PART 3 PART 4 • FOUR WAYS TO COOK VEGETABLES • TAKE A LOOK AT YOUR PLATE —MAKING RECIPES LIVER FRIENDLY • SAMPLE MEAL PLAN • GROCERY SHOPPING AND PLANNING MEALS • SNACKS & SMOOTHIES PART PART 1 THE LIVER AND NAFLD WHERE IS THE LIVER? The Iiver is one of the largest organs in the body. It is located in the upper right side of the belly. The liver is the only organ able to repair itself after injury. Your liver is essential to your life. You cannot live without it. THE LIVER— ASSISTS IN DIGESTION OF PROTEINS, FATS AND CARBOHYDRATES IS ESSENTIAL FOR DEVELOPING PROTEINS THAT SUPPORT OUR MUSCLE AND IMMUNE SYSTEM STORES AND RELEASES ENERGY, VITAMINS AND MINERALS HELPS MAINTAIN BLOOD SUGAR REMOVES TOXINS FROM THE BLOOD STREAM 2 WHAT IS NON-ALCOHOLIC FATTY LIVER DISEASE? Non-Alcoholic Fatty Liver Disease (NAFLD) results when liver cells become filled with fat. This fat can cause injury to the liver which can lead to cirrhosis, a severe condition where the liver is permanently scarred and damaged. Cirrhosis can lead to liver cancer or liver failure. You can control the progression of NAFLD by making the choice to change your diet and engage in daily activity. Most people do not know About 30% of U.S adults they have NAFLD, signs and have non-alcoholic fatty liver symptoms do not appear until disease. -
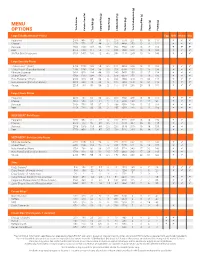
Menu Options
MENU OPTIONS Calories Total Fat Calories from (g) Fat Total (g) Fat Saturated (g) Fat Trans (mg) Cholesterol Sodium (mg) (g) Carbohydrate Total Dietary Fiber (g) (g) Sugars (g) Protein Large ExtraMostBestest® Pizzas Egg Milk Wheat Soy Pepperoni 2470 990 112 48 3.5 270 5570 251 13 14 117 a a a Cheese 2270 770 87 44 3 225 4490 253 13 12 121 a a a Sausage 2650 1130 128 53 2.5 290 5860 252 13 12 126 a a a Beef 2550 1030 117 52 5 280 5620 250 13 12 126 a a a Stuffed Crust Pepperoni 3030 1400 158 72 4.5 395 7170 259 13 15 146 a a a Large Specialty Pizzas 5 Meat Feast™ (Ham) 2730 1180 133 56 3.5 315 6600 252 13 15 133 a a a 5 Meat Feast (Canadian Bacon) 2740 1190 134 56 3.5 320 6740 252 13 15 134 a a a Ultimate Supreme 2410 920 104 44 2.5 240 5450 258 15 17 114 a a a 3 Meat Treat® 2760 1230 139 58 3 310 6300 252 13 14 130 a a a Hula Hawaiian® (Ham) 2160 610 69 32 2 200 4960 272 14 30 114 a a a Hula Hawaiian (Canadian Bacon) 2200 640 72 33 2 215 5330 273 13 31 116 a a a Veggie 2250 740 84 39 2 155 5310 266 20 19 101 a a a Large Classic Pizzas Pepperoni 2210 790 90 39 2.5 210 4660 249 13 13 105 a a a Cheese 1950 580 65 31 2 150 3680 248 12 12 95 a a a Sausage 2160 750 85 37 2 190 4350 249 12 12 104 a a a Beef 2150 740 83 38 3 195 4350 248 13 12 106 a a a DEEP!DEEP!™ Dish Pizzas Pepperoni 2770 980 111 47 3 240 5220 319 16 16 129 a a Cheese 2500 750 85 38 2.5 175 4170 317 16 14 118 a a Sausage 2810 1010 114 48 2.5 235 5170 319 16 14 131 a a Beef 2750 950 107 47 3.5 230 5010 318 16 14 130 a a DEEP!DEEP!™ Dish Specialty Pizzas Ultimate Supreme 3050 -

Original.Pdf
ffERRINGS SALT PICКLING Herring barrel cream-soaked 280 AND VINEGAR PICКLE Baltic herring 260 Soft-salted cabbage 380 Dressed with beet-root 360 Fermented cabbage 380 п mustard sauce 320 Provensal cabbage 380 Wine-marinated 340 Pickled cucumbers 380 Herring cold smoked 280 Guryiskaya cabbage 380 Big Caspian herring 490 Soft-salted cucumbers 380 Pickled ог soft-salted tomatoes 380 SALADS AND SNACKS Cherry tomatoes 380 Jelly of 3 types of meat 380 Pickled apples 380 Carpaccio of duck with turnip апd sea buckthorn 460 Salt mushrooms (100g) Согпеd beef with honey-mustard sauce 450 280 Tartarian beef with pickled cucumber 460 Теlпоуе of сагр 980 Mushrooms in sour cream 420 Ears of chanterelle 400 CAVIAR The burbot liver with опiоп marmalade 650 Cod liver with mustard dressing 420 Pressed sturgeon (50gr) 2600 Home salad with fragrant oil 280 Black sturgeon (100gr) 2600 Russian salad "Zhivago" with pickled cucumber 370 Russian salad Lucien 750 Red trout 750 Vinegret of КuЬап tomatoes with Yalta опiоп 380 Red salmon 650 Greens salad with salmon апd spinach 580 Mushroom Paste COLD CUTS Salad with пеw potatoes апd cream, 320 red caviar апd dill Eggplant Paste 300 Salmon far East 560 490 СгаЬ salad with oranges and pike гое 650 Pike 580 Salad with snake-root knotgrasses, arugula and pumpkin Salmon smoked 490 560 Herring in а pancake Sturgeon fillets 550 Salad with roots with dried cranberries 280 Salad with roast beef, arugula and raspberries 590 with scallions 250 Nelma 550 Warm salad with chicken umЫes апd wild partridge Ьеггу 380 Chir450 Omul -

Food in Pregnancy
Food in Pregnancy A healthy diet is an important part of a healthy lifestyle at any time, but is especially vital if you're pregnant. Eating healthily during pregnancy will help your baby to develop and grow. You will probably find that you are hungrier than usual, but you don't need to "eat for two" – even if you are expecting twins or triplets as this can cause weight gain which might be difficult to shed later. Having healthy snacks like fruit and vegetable sticks with you will make it easier to keep hunger at bay. There are some foods to avoid or take care with when you're pregnant, because they might make you ill or harm your baby. Make sure you know the important facts about which foods you should avoid or take precautions with when you're pregnant………… Raw or undercooked meat Fish Do not eat raw or undercooked meat, including meat Cheese Meat & Eggs joints and steaks cooked rare, because of the potential Fish to avoid: risk of toxoplasmosis. Cook all meat and poultry Cheese thoroughly so it's steaming hot and there's no trace of When you're pregnant or planning to get pregnant, you shouldn't eat pink or blood – especially with poultry, pork, sausages Don't eat mould-ripened soft cheese (cheeses with shark, swordfish or marlin. and minced meat, including burgers. Raw or undercooked meat a white rind) such as brie and camembert. This includes mould-ripened soft goats' cheese, such as Toxoplasmosis is an infection caused by a parasite Do not eat raw or undercooked meat, including meat joints and steaks Fish to restrict: chevre. -

Restaurants to the Rescue Lettuce, Tomatoes and Mayo
40. WESTOVER CLUB - Hickory 47. THE HOUND DOG - Chicken LOST DOG SPECIALTY SANDWICHES ham, turkey breast, bacon and provolone cheese with barbecue served with cole slaw and melted Swiss on Restaurants to the Rescue lettuce, tomatoes and mayo. 8.50 a toasted roll. 8.50 ADD POTATO CHIPS & PICKLE FOR 95¢ • ADD ITALIAN FRIES FOR $2.95 • ADD DOG COLLARS FOR $3.95 The Café – The Lost Dog story begins in a tiny storefront space in 41. CALIFORNIA DREAMING 48. LOLA LAB - Homemade black Whole wheat sub roll stuffed with our lean spiced bean dip, melted cheddar and mozzarella cheeses Arlington’s Westover neighborhood in 1985. That’s when Ross Underwood 1. HCBLT - Hot hickory ham, melted provolone 21. THE PHOENIX - Tender chunks of turkey breast with lettuce, tomatoes, alfalfa sprouts topped with shredded lettuce, pico de gallo and fresh cheese, bacon, lettuce, tomatoes and mayo. 8.50 seasoned chicken breast baked and placed in pita with and avocado. 8.50 avocado served on a warm pita. 8.50 and Pam McAlwee pooled every penny they could scrounge to open the melted mozzarella cheese, lettuce and garlic mayo. 8.50 2. BILLY'S PHILLY - Char-grilled 42. MUTTLY - Warm pita stuffed with 50. POUND HOUND - Three wine and cheese shop that soon became the Lost Dog Café. They started chicken breast, melted cheddar cheese, lettuce, tomatoes 22. SEA DOG SALAD - Backfin crabmeat, grilled chicken breast, pesto, tomatoes, spinach, layers of French toast, turkey breast, hickory ham small, dreamed big and worked countless long hours until things slowly fell and garlic mayo on a toasted sub roll. -

See Our Menu
765-354-9880 MONDAY—THURSDAY: 11 a.m. – 10 p.m., Pepperoni’s Pizza FRIDAY & SATURDAY: 11 a.m. – 11 p.m., apa 594 Locust St., Middletown DOWNLOAD SUNDAY: 11 a.m. – 9 p.m. P SALES TAX AND DELIVERY FEES MAY APPLY. OUR PHONE APP! PIZZAS & 8” 10” 12” 14” 16” 20” CALZONES 4 Slices 6 Slices 8 Slices 10 Slices 12 Slices 16 Slices Just Cheese $3.99 $4.99 $8.99 $10.99 $12.99 $17.99 1 Topping $4.74 $5.99 $10.24 $12.49 $14.99 $20.99 2 Topping $5.49 $6.99 $11.49 $13.99 $16.99 $23.99 Extra Toppings Ea. 75¢ $1.00 $1.25 $1.50 $2.00 $3.00 Specialties $6.99 $8.99 $13.99 $17.99 $20.99 $29.99 Calzones Just Cheese $5.99 Specialty $9.99 Add Toppings Ea. $1 Gluten-Free All 10” pizzas can be made gluten-free for only $1.00 more PAPA PEPPERONI’S SPECIALTY PIZZAS DELUXE: Pepperoni, sausage, green 6 CHEESE: Mozzarella, Muenster, MEAT EATERS: Pepperoni, ham, peppers, onions, black olives, mushrooms, cheese. Provolone, Cheddar, Asiago, & Parmesan Italian sausage, bacon, ground beef, cheese. cheese. CHICKEN CARBONARA: CHICKEN BACON Alfredo sauce, chicken, mushrooms, bacon, SANTA FE CHICKEN (SPICY!): Chicken, ranch, bacon, cheese. cheese. Chipotle ranch, chicken, onions, bacon, RANCH: jalapenos, cheese. TRIPLE PEPPERONI: Three HAWAIIAN: Ham, pineapple, BBQ kinds of pepperoni (and lots of it!), cheese. VEGGIE: Mushrooms, green peppers, sauce, cheese. onions, black olives, tomato, banana peppers, BBQ CHICKEN: Sweet BBQ, cheese. SPINACH ALFREDO: Alfredo, chicken, onions, green peppers, cheese. -

STUFFED CRUST All Our Burgers Are Served KEBABS MAKE IT MED LRG BURGERS with Chips, Salad & Sauce
THIN CRUST DEEP PAN STUFFED CRUST All our burgers are served KEBABS MAKE IT MED LRG BURGERS with chips, salad & sauce. 1/4lb 1/2lb ADD £2.20 DONER KEBAB meal! 4.90 6.30 BEEF BURGER 4.40 5.50 lamb meat cooked on a spit FOR CHIPS & DRINK CHEESE BURGER 4.60 5.70 CHICKEN DONER KEBAB 4.90 6.30 chicken meat cooked on a spit 9” 12” 15” DOUBLE BACON & CHEESE BURGER 5.40 6.50 MIXED DONER lamb & chicken meat cooked on a spit 5.30 6.50 PIZZAS 6 SLICES 8 SLICES 12 SLICES CHICKEN BURGER 4.40 5.50 We don’t use frozen dough for our pizza, we make it fresh on our premises everyday. CHICKEN SHISH KEBAB 5.30 7.50 All our toppings are freshly prepared every morning! CHICKEN FILLET BURGER ADD 5.10 6.50 chicken breast marinated with special sauce MARGHERITA tomato sauce & mozzarella cheese 8.10 10.70 12.80 VEGGIE BURGER 4.40 5.40 LAMB SHISH KEBAB 5.60 7.90 ham & pineapple FISH BURGER drink! 4.40 5.40 lamb meat marinated with special sauce HAWAIIAN WITH ANY BURGER KOFTE KEBAB 5.00 6.80 FARMHOUSE ham & mushrooms 9.30 12.30 14.60 PINEAPPLE BURGER OR SPECIAL BURGER 5.00 6.00 fresh minced lamb with parsley TUNA & SWEETCORN tuna & sweetcorn BACON ROLL 3pcs of bacon only 70p 4.00 - & oriental herbs cooked on a grill PEPPERONI LOVER 10.00 13.10 15.60 DONER ROLL chicken or lamb 4.50 - CHICKEN TIKKA KEBAB 5.60 7.60 cheese & double pepperoni MEAT & CHIPS 4.90 6.30 KIEV PIZZA chicken, ham & garlic chicken or lamb doner with chips, served without pitta VEGETARIAN mushrooms, onions, 10.70 14.00 16.60 SPECIAL BURGERS MIXED MEAT & CHIPS 5.30 6.50 green peppers, fresh tomatoes & sweetcorn All our burgers are served with chips, salad & sauce. -

Np 093 16.Pdf
• • / I , .++• Greater Newark's Hometown Newspaper Since 1910 .++• 93rd Year, Issue 16 ©2002 May 8,2002 Newark, Delaware • SOt Getting Newark celebrates its Newark girls better all tie St. Mark's. the time. downtown. - - PAGE :s PACE ti - ~,.~-~-----=""~~~~~~~ - ~~----- - - .. Up FRONT Kicking back with Getreadl Some' bits tea on Mom's Day Judge Morris Estate showing to be and pieces off wildflowers and garden By JIM STREIT By CHRISTINE E. SERIO patient . NEWARK POST STAFF WRITER NEWARK POST STAFF WRITER HE FIRST of the month Tfinds me rummaging Delays expected as work through the stack of OMS CAN TAKE A BREAK from juggling careers Post-Its cluttering my desk. and family and enjoy a variety of tea parties planned begins on Library Avenue, Like the tornado that hit Cecil for Mother's Day this year. County last week, I'm dealing Sipping tea in a garden may be one way to give mom the cel road closures begin mid-June with them: ebration she deserves. Staff at White Clay Creek State Park • Last week's ranting Nature Center in Newark will host a Wildflower Walk and Tea at By MARY E. PETZAK about baseball and my memo the Judge Morris Estate on Polly Drummond Hill Road. ries of riding the streetcar to Daughters or sons can treat mom at 10 a.m. on Saturday or 1 NEWARK POST STAFF WRITER Baltimore Orioles spurred an p.m, on Sunday at the historic home built in the late 1700s and interesting visit last week. once lived in by Judge Hugh Morris, a Delaware judge and a on Library AvenuelRoute 72 in Newark, designated the next Carl H.