Optic Neuropathy Associated with Systemic Sarcoidosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Case of Anterior Ischemic Optic Neuropathy Associated with Uveitis
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT A case of anterior ischemic optic neuropathy associated with uveitis Michitaka Sugahara Introduction: Here, we describe a patient who presented with anterior ischemic optic Takayuki Fujimoto neuropathy (AION) and subsequently developed uveitis. Kyoko Shidara Case: A 69-year-old man was referred to our hospital and initially presented with best-corrected Kenji Inoue visual acuities (BCVA) of 20/40 (right eye) and 20/1000 (left eye) and relative afferent pupillary Masato Wakakura defect. Slit-lamp examination revealed no signs of ocular inflammation in either eye. Fundus examination revealed left-eye swelling and a pale superior optic disc, and Goldmann perimetry Inouye Eye Hospital, Tokyo, Japan revealed left-eye inferior hemianopia. The patient was diagnosed with nonarteritic AION in the left eye. One week later, the patient returned to the hospital because of vision loss. The BCVA of the left eye was so poor that the patient could only count fingers. Slit-lamp examination revealed 1+ cells in the anterior chamber and the anterior vitreous in both eyes. Funduscopic examination revealed vasculitis and exudates in both eyes. The patient was diagnosed with bilateral panuveitis, and treatment with topical betamethasone was started. No other physical findings resulting from other autoimmune or infectious diseases were found. No additional treatments were administered, and optic disc edema in the left eye improved, and the retinal exudates disappeared in 3 months. The patient’s BCVA improved after cataract surgery was performed. Conclusion: Panuveitis most likely manifests after the development of AION. -

Ocular Dysmetria in a Patient with Charcot-‐Marie-‐ Tooth Disease
Ocular Dysmetria in a Patient with Charcot-Marie- Tooth Disease Michelle Lee, OD A patient with the inherited neuropathy, Charcot-Marie-Tooth disease (CMT), presents with ocular dysmetria. Although abnormal ocular motility has not been reported in CMT patients, the absence of other etiologies indicates a possible ocular manifestation. CASE HISTORY • Patient demographics: 74 year old Caucasian male • Chief complaint: no visual or ocular complaints • Ocular History o Mild cataracts OU o Dry eye syndrome OU o Refractive error OU • Medical history o Charcot-Marie-Tooth disease o Asthma o Hypercholesterolemia o Herpes zoster o Chronic lower bacK pain o Dermatitis o Obstructive sleep apnea • Medications o Albuterol o Gabapentin o Meloxicam o Mometasone furoate o Oxybutynin chloride o Simvastatin o Tamusolisn HCL o Aspirin o Vitamin D • Ocular medications: artificial tears prn OU • Family history: father and grandfather also with CMT PERTINENT FINDINGS • Clinical o Mixed hypometric and hypermetric saccades with intermittent disconjugate movement o Trace restriction of lateral gaze and inferior temporal OS o Ptosis OD o Borderline reduced contrast sensitivity OD, mildly reduced contrast sensitivity OS o Pertinent negatives: no evidence of light-near-dissociation, no signs of optic neuropathy 1 of 4 • Physical o Abnormal gait • Lab studies o EMG consistent with positive family history of CMT • Radiology studies o MRI (04/13): no intracranial mass or acute infarcts seen, no evidence of cerebellar abnormality noted DIFFERENTIAL DIAGNOSIS • Primary/leading -

Ocular Side Effects of Systemic Drugs.Cdr
ERA’S JOURNAL OF MEDICAL RESEARCH VOL.6 NO.1 Review Article OCULAR SIDE EFFECTS OF SYSTEMIC DRUGS Pragati Garg, Swati Yadav Department of Ophthalmology Era's Lucknow Medical College & Hospital, Sarfarazganj Lucknow, U.P., India-226003 Received on : 06-03-2019 Accepted on : 28-06-2019 ABSTRACT Systemic drugs are frequently administered in persons of all age group Address for correspondence ranging from children to the elderly for various disorders. There has been Dr. Pragati Garg increased reporting of ocular side effects of various systemic drugs in the Department of Ophthalmology past two decades. Some offenders well known are α -2-adrenergic agonists, Era’s Lucknow Medical College & quinine derivatives, β- adrenergic antagonists and antituberculosis drugs. Hospital, Lucknow-226003 Newer systemic drugs causing ocular side effects are being reported in Email: [email protected] available literature. Knowledge regarding these is expected to aid Contact no: +91-9415396506 clinicians in identifying these side effects and the offending drug, thereby, prescribing the appropriate treatment for the condition the patient maybe suffering from without any ocular disturbances. KEYWORDS: Ocular side effects, Systemic drugs. Introduction This article will briefly cover how systemic drugs can Many common systemic medications can affect ocular affect the various ocular structures. tissues and visual function to varying degrees. When a Factors Affecting The Production Of Ocular Side systemic medication is taken to treat another part of the Effects By A Drug body, the eyes frequently are affected. Systemic A) Drug related factors medications can have adverse effects on the eyes that range from dry eye syndrome, keratitis and cataract to (1) The nature of the drug: Absorption of drug in blinding complications of toxic retinopathy and optic body and its pharmacological effects on the body's neuropathy (1). -

Acquired Pendular Nystagmus in Multiple Sclerosis: Clinical Observations and the Role of Optic Neuropathy 263
262 journal ofNeurology, Neurosurgery, and Psychiatry 1993;56:262-267 Acquired pendular nystagmus in multiple J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.56.3.262 on 1 March 1993. Downloaded from sclerosis: clinical observations and the role of optic neuropathy Jason J S Barton, Terry A Cox Abstract identified from the files of patients seen Thirty seven patients with pendular nys- between 1981-90 at the MS Clinic at the tagmus due to multiple sclerosis were University of British Columbia. Only those reviewed. Most developed nystagmus with a "clinically definite" or "clinically prob- later in a progressive phase of the dis- able" diagnosis of MS4 and who had been ease. All had cerebellar signs on exami- examined by a neuro-ophthalmologist were nation and evidence of optic neuropathy. accepted. Two patients were not studied fur- MRI in eight patients showed cerebeliar ther because of insufficient data. or brainstem lesions in seven; the most Data were taken from the first neuro-oph- consistent finding was a lesion in the dor- thalmologic examination noting pendular nys- sal pontine tegmentum. Dissociated nys- tagmus to document the signs most closely tagmus was seen in 18 patients: in these associated with its appearance. Visual acuity the signs of optic neuropathy were often after refraction was assessed with projected asymmetric and the severity correlated Snellen charts. Colour vision was scored with closely with the side with larger oscilla- 16 Ishihara pseudo-isochromatic plates and tions. This suggests that dissociations in optic atrophy was graded on fundoscopy on a acquired pendular nystagmus may be scale of 0 to 4.5 Ocular motility and the due to asymmetries in optic neuropathy amplitude and trajectory of pendular nystag- rather than asymmetries in cerebellar or mus were assessed clinically. -

Pediatric Neuro-Ophthalmology
Pediatric Neuro-Ophthalmology Second Edition Michael C. Brodsky Pediatric Neuro-Ophthalmology Second Edition Michael C. Brodsky, M.D. Professor of Ophthalmology and Neurology Mayo Clinic Rochester, Minnesota USA ISBN 978-0-387-69066-7 e-ISBN 978-0-387-69069-8 DOI 10.1007/978-0-387-69069-8 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010922363 © Springer Science+Business Media, LLC 2010 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connec-tion with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with re-spect to the material contained herein. Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) To the good angels in my life, past and present, who lifted me on their wings and carried me through the storms. -

Amaurosis Fugax (Transient Monocular Or Binocular Vision Loss)
Amaurosis fugax (transient monocular or binocular vision loss) Syndee Givre, MD, PhD Gregory P Van Stavern, MD The next version of UpToDate (15.3) will be released in October 2007. INTRODUCTION AND DEFINITIONS — Amaurosis fugax (from the Greek "amaurosis," meaning dark, and the Latin "fugax," meaning fleeting) refers to a transient loss of vision in one or both eyes. Varied use of common terminology may cause some confusion when reading the literature. Some suggest that "amaurosis fugax" implies a vascular cause for the visual loss, but the term continues to be used when describing visual loss from any origin and involving one or both eyes. The term "transient monocular blindness" is also often used but is not ideal, since most patients do not experience complete loss of vision with the episode. "Transient monocular visual loss" (TMVL) and "transient binocular visual loss" (TBVL) are preferred to describe abrupt and temporary loss of vision in one or both eyes, since they carry no connotation regarding etiology. Transient visual loss, either monocular or binocular, reflects a heterogeneous group of disorders, some relatively benign and others with grave neurologic or ophthalmologic implications. The task of the clinician is to use the history and examination to localize the problem to a region in the visual pathways, identify potential etiologies, and, when indicated, perform a focused battery of laboratory tests to confirm or exclude certain causes. Therapeutic interventions and prognostic implications are specific to the underlying cause. This topic discusses transient visual loss. Other ocular and cerebral ischemic syndromes are discussed separately. APPROACH TO TRANSIENT VISUAL LOSS — By definition, patients with transient visual loss almost always present after the episode has resolved; hence, the neurologic and ophthalmologic examination is usually normal. -
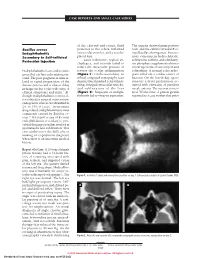
Leber's Hereditary Optic Neuropathy Masquerading As Retinal Vasculitis
CASE REPORTS AND SMALL CASE SERIES of the choroid and retina, fluid The aspirate showed gram-positive Bacillus cereus posterior to the sclera, inflamed rods, and the culture revealed B ce- Endophthalmitis extraocular muscles, and a nondis- reus/Bacillus thuringiensis. Intrave- Secondary to Self-inflicted placed lens. nous vancomycin hydrochloride, Laser iridotomy, topical cy- ceftriaxone sodium, and clindamy- Periocular Injection cloplegics, and steroids failed to cin phosphate supplemented intra- reduce the intraocular pressure or vitreal injections of vancomycin and Endophthalmitis is an ocular emer- reverse the ocular inflammation ceftazidime. A normal echocardio- gency that can have a devastating out- (Figure 1). On the second day, an gram ruled out a cardiac source of come. The poor prognosis is often re- orbital computed tomography scan bacteria. On the fourth day, spon- lated to rapid progression of the demonstrated marked scleral thick- taneous scleral perforation oc- disease process and a relative delay ening, enlarged extraocular muscles, curred with extrusion of purulent in diagnosis due to the wide array of and subluxation of the lens uveal contents. The eye was eviscer- clinical symptoms and signs.1 Al- (Figure 2). Suspicion of endoph- ated. Weeks later, 2 prison guards though endophthalmitis is most of- thalmitis led to vitreous aspiration. reported to a case worker that prior ten related to surgical intervention, endogenous sources are identified in 2% to 15% of cases.1 Intravenous drug-related endophthalmitis is most commonly caused by Bacillus ce- reus.2,3 We report a case of B cereus endophthalmitis secondary to peri- orbital drug injection that resulted in spontaneous lens subluxation. -

Churg-Strauss Vasculitis Presenting with Severe Visual Loss Due to Bilateral Sequential Optic Neuropathy
18 BritishJournal ofOphthalmology: case reports CASE REPORTS Br J Ophthalmol: first published as 10.1136/bjo.77.2.118 on 1 February 1993. Downloaded from Churg-Strauss vasculitis presenting with severe visual loss due to bilateral sequential optic neuropathy J F Acheson, 0 C Cockerell, C R Bentley, M D Sanders Abstract family had any eye disease. His medical history A 44-year-old man with severe visual loss consisted of late onset asthma diagnosed 5 years due to an acute bilateral sequential optic earlierwhenhehaddevelopedepisodicwheezing, neuropathy is described, where the associated cough, and dyspnoea requiring multiple hospital pulmonary disease and peripheral eosinophilia admissions. His respiratory symptoms were led to a diagnosis of Churg-Strauss syndrome partially controlled at the time ofvisual loss with (allergic angiitis). The mechanism of the optic oral prednisolone 40 mg daily and aerosol neuropathy wasmostprobably acute ischaemia bronchodilators. ofthe anterior optic nerve due to direct involve- On examination his right visual acuity was no ment of the short posterior ciliary arteries by perception of light and the left eye saw counting inflammatory disease ofthe vessel wall. fingers. The eye movements, anterior segments, (BrJ Ophthalmol 1993; 77: 118-119) and intraocular pressure were normal. There was no afferent pupillary light reaction on the right and a reduced response on the left. The left eye In 1951 Churg and Strauss first reported 13 cases had no colour perception and Goldmann of adult onset asthma followed by systemic perimetry showed superior and inferior arcuate vasculitis associated with peripheral eosino- defects with peripheral depression leaving a philia.' Histologically there was evidence of residual 10 degree field. -

Ischemic Optic Neuropathy Raman Bahkhri, OD Ischemic Optic Neuropathy Can Potentially Be a Visually Devastating Condition Among Middle- Aged and Older Individuals
Ischemic optic neuropathy Raman Bahkhri, OD Ischemic optic neuropathy can potentially be a visually devastating condition among middle- aged and older individuals. It can be divided into anterior ischemic optic neuropathy (AION) and posterior ischemic optic neuropathy (PION) based on the anatomical vascular supply of the optic nerve head that is afflicted. AION is then further classified as either arteritic (A-AION), commonly caused either by giant cell arteritis (GCA), or non arteritic (NA-AION) with multiple causes other than giant cell. Likewise, PION has two subclasses in addition to a surgical classification (Figure 1). The most common of these conditions is NA-AION with PION being the rarest. This discussion will review the clinical presentation, pathogenesis, work up, prognosis and treatment of these neuropathies. A-AION The primary cause of A-AION is GCA although other conditions such as polyarteritis nodasa, CE@Home polymyalgia rheumatica, lupus and herpes zoster have also been known to cause A-AION. GCA is a type of vasculitis and has a predilection for medium and large size arteries, specifically the posterior ciliary arteries (PCA), which supply the anterior portion of the optic nerve. Conse- quently, this leads to the formation of a thrombotic occlusion of the PCA, thus causing an infarction of the anterior portion of the optic nerve.1 Dr. Raman Bhakhri is an Patients affected by A-AION present with acute unilateral vision loss with mean visual acuity assistant professor at the of 20/400, to no light perception.2 The average age of patients is 76 years old with women Southern California College (70 percent) being affected more often than men (30 percent). -

Papilledema": Neuroradiologic Evaluation of Optic Disk Protrusion with Dynamic Orbital CT
681 "Papilledema": Neuroradiologic Evaluation of Optic Disk Protrusion with Dynamic Orbital CT John R. Jinkins 1 Current-generation CT scc·lners enable the visualization in vivo of structures and substructures that were previously unobservable. Certainly the orbit and optic nerve/ sheath complex have demonstrated a great number of pathologic and normal anatomic variations. It has been found in patients with elevated intracranial pressure that what was previously thought to be simple papilledema in fact masks a surprisingly large component of optic papilla protru<;ion, There may be a variable amount of increased intercellular/axonal fluid within tt.. - optic disk in patients with increased intracranial pressure; however, a significant factor in the " swollen disk" is the simple transmission of pressure along the optic nerve sheath to the papilla, causing it to bulge. Further investigations with dynamic CT reveal that there is decreased perfusion of the optic disk in the active phase of severe increased intracranial pressure in patients with papilledema and/or protrusion as compared with normal control subjects. This de pressed flow pattern seems to originate subacutely and appears to resolve in certain patients after normalization of the elevated pressure. These findings apparently indicate that clinical intervention in cases of intracranial hypertension to restore the hemodynamic status of the optic disk would be timely, and thereby avert irreversible damage. This suggests and supports the theory that increased intracranial pressure may lead to rapid vision loss by the mechanical mechanism of pressure projected directly to the junction of the optic nerve and optic nerve head, leading to decreased perfusion, ischemia, axonal flow stasis, and resultant optic nerve atrophy. -

The 2017 Doyne Lecture: the Orbit As a Window to Systemic Disease
Eye (2018) 32, 248–261 © 2018 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 0950-222X/18 www.nature.com/eye REVIEW The 2017 Doyne AA McNab Lecture: the orbit as a window to systemic disease Abstract A very large number of disorders affect the associations. I will not cover these in orbit, and many of these occur in the setting of encyclopaedic detail, but will instead focus on systemic disease. This lecture covers selected aspects of orbital disease with systemic aspects of orbital diseases with systemic asso- associations that have been of particular clinical ciations in which the author has a particular and research interest to me. clinical or research interest. Spontaneous orbital haemorrhage often occurs in the presence of Orbital vascular disease bleeding diatheses. Thrombosis of orbital veins and ischaemic necrosis of orbital and ocular Two generations ago, Duke-Elder’s multi- adnexal tissues occur with thrombophilic dis- volume textbook was on every orders, vasculitis, and certain bacterial and ophthalmologist’s bookshelf. Volume 13, part 2 fungal infections. Non-infectious orbital inflam- of this seminal work has a section on mation commonly occurs with specificinflam- spontaneous orbital haemorrhage.1 Under ’ matory diseases, including Graves disease, haemorrhagic diatheses, it states ‘the most ’ IgG4-related disease, sarcoidosis, Sjögren ssyn- important are haemophilia and scurvy’. I doubt drome and granulomatosis with polyangiitis, all any of you see cases of either disease now. But of which have systemic manifestations. IgG4- scurvy is an incredibly important disease, which related ophthalmic disease is commoner than all profoundly influenced world history. More ’ these except Graves orbitopathy. -

Sarcoidosis Optic Neuropathy
Sarcoidosis Optic Neuropathy Optometric Retina Society Residency Award Delia Groshek, OD White River Junction VA Medical Center Ocular Disease Residency 2010-2011 6244 North Knox Avenue Chicago, IL 60646 (773) 620-5993 [email protected] Abstract Sarcoidosis is a multi-systemic granulomatous disease of unknown etiology that can affect almost every organ in the body, though it typically affects the lung, lymph nodes, skin, liver and eyes. The diagnosis of sarcoidosis is based on histological evidence of noncaseating epithelioid cell granulomas, bilateral hilar lymphadenopathy, and exclusion of other diseases that produce a similar clinical and/or histological picture. Ocular involvement may be the presenting sign of the disease and can involve any ocular structure. In this case, a 67 year old Caucasian male presents with blur, photopsia, papillitis, and periphlebitis. This case report reviews the diagnosis, treatment and management of ocular sarcoidosis. Key words: angiotension converting enzyme, bilateral hilar lymphadenopathy, noncaseating granuloma, papillitis, periphlebitis, sarcoidosis Case Report A 67 year old Caucasian male presented to the VA eye clinic with a chief complaint of central blurring of his vision in his left eye, which happened twice the previous night lasting for five minutes each time. Afterwards, he saw blue spots. In the exam, he stated his left eye felt like it had a dull, aching pain of a 2-3 on a scale of 10. He reported itching and tearing of his left eye. Upon further questioning, the patient denied scalp tenderness, jaw claudication, or recent weight loss. The patient did report neck pain, which has been stable for years due to his arthritis.