Evolution in the Weak-Mutation Limit: Stasis Periods Punctuated by Fast Transitions Between Saddle Points on the Fitness Landscape
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Punctuated Equilibrium Theory Variations in Punctuated Equilibrium and and the Diffusion of Innovation
An Introduction to Punctuated Equilibrium: A Model for Understanding Stability and Dramatic Change in Public Policies January 2018 This briefing note belongs to a series on the Tobacco policies can serve as an example to various models used in political science to illustrate this idea. Up until 1965, this policy had represent public policy development processes. changed very little, whereas in the late 1960s and Each of these briefing notes begins by describing early 1970s a radical change occurred in the analytical framework proposed by the given response to the actions of certain stakeholders, model. With this model in mind, we then set out to such as the US Surgeon General's 1964 examine questions that public health actors might publication of the now-famous report entitled ask about public policies. Our aim in these notes Smoking and Health. is not to further refine existing models; nor is it to advocate for the adoption of one model in To incorporate their insight into public policy particular. Our purpose is rather to suggest how analysis, Baumgartner and Jones sought to each of these models constitutes a useful reconcile in an integrated model the long periods interpretive lens that can guide reflection and of equilibrium, already well explained by the action leading to the production of healthy public incrementalist model, and the abrupt policies. punctuations of political systems. This became known as the punctuated equilibrium model. The punctuated equilibrium model aims to explain why public policies tend to be characterized by long periods of stability punctuated by short periods of radical change. -

What Can We Learn from Fitness Landscapes?
What can we learn from fitness landscapes? The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Hartl, Daniel L. 2014. “What Can We Learn from Fitness Landscapes?” Current Opinion in Microbiology 21 (October): 51–57. doi:10.1016/j.mib.2014.08.001. Published Version 10.1016/j.mib.2014.08.001 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:22898356 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP Elsevier Editorial System(tm) for Current Opinion in Microbiology Manuscript Draft Manuscript Number: Title: What Can We Learn From Fitness Landscapes? Article Type: 22 Growth&Develop: prokaryotes (2014 Corresponding Author: Dr. Daniel Hartl, Corresponding Author's Institution: First Author: Daniel Hartl Order of Authors: Daniel Hartl Manuscript Click here to view linked References What Can We Learn From Fitness Landscapes? Daniel L. Hartl Department of Organismic and Evolutionary Biology Harvard University Cambridge, Massachusetts 02138 USA Contact information: Email: [email protected], TEL: 617-396-3917 In evolutionary biology, the fitness landscape of set of mutants is the mapping of genotypes onto phenotypes when the phenotype is fitness or some proxy for fitness such as growth rate or drug resistance. When the set of mutants is not too large, it is possible to create every possible combination of mutants and map these to fitness. -

1. Basis of Fitness Landscape
Optimisation Origin and definition of fitness landscape Position and goal 1. Basis of fitness landscape Fitness landscape analysis for understanding and designing local search heuristics Sebastien´ Verel LISIC - Universit´edu Littoral C^oted'Opale, Calais, France http://www-lisic.univ-littoral.fr/~verel/ The 51st CREST Open Workshop Tutorial on Landscape Analysis University College London 27th, February, 2017 Optimisation Origin and definition of fitness landscape Position and goal Outline of this part Basis of fitness landscape : introductory example (Done) brief history and background of fitness landscape fundamental definitions Optimisation Origin and definition of fitness landscape Position and goal Mono-objective Optimization Search space : set of candidate solutions X Objective fonction : quality criterion (or non-quality) f : X ! IR X discrete : combinatorial optimization X ⊂ IRn : numerical optimization Solve an optimization problem (maximization) ? X = argmaxX f or find an approximation of X ?. Optimisation Origin and definition of fitness landscape Position and goal Context : black-box optimization x −! −! f (x) No information on the objective definition function f Objective fonction : can be irregular, non continuous, non differentiable, etc. given by a computation or a simulation Optimisation Origin and definition of fitness landscape Position and goal Real-world black-box optimization : first example PhD of Mathieu Muniglia, Saclay Nuclear Research Centre (CEA), Paris x −! −! f (x) (73;:::; 8) −! −! ∆z P Multi-physic simulator Optimisation Origin and definition of fitness landscape Position and goal Search algorithms Principle Enumeration of the search space A lot of ways to enumerate the search space Using exact method : A?, Branch&Bound, etc. Using random sampling : Monte Carlo technics, approx. -

V Sem Zool Punctuated Equilibrium
V Sem Zool Punctuated Equilibrium Gradualism and punctuated equilibrium are two ways in which the evolution of a species can occur. A species can evolve by only one of these, or by both. Scientists think that species with a shorter evolution evolved mostly by punctuated equilibrium, and those with a longer evolution evolved mostly by gradualism. Both phyletic gradualism and punctuated equilibrium are speciation theory and are valid models for understanding macroevolution. Both theories describe the rates of speciation. For Gradualism, changes in species is slow and gradual, occurring in small periodic changes in the gene pool, whereas for Punctuated Equilibrium, evolution occurs in spurts of relatively rapid change with long periods of non-change. The gradualism model depicts evolution as a slow steady process in which organisms change and develop slowly over time. In contrast, the punctuated equilibrium model depicts evolution as long periods of no evolutionary change followed by rapid periods of change. Both are models for describing successive evolutionary changes due to the mechanisms of evolution in a time frame. Punctuated equilibrium The punctuated equilibrium hypothesis states that speciation events occur rapidly in geological time - over hundreds of thousands to millions of years and that little change occurs in the time between speciation events. In other words, change only happens under certain conditions, and it happens rapidly. Instead of a slow, continuous movement, evolution tends to be characterized by long periods of virtual standstill or equilibrium punctuated by episodes of very fast development of new forms. It was proposed by Eldridge and Gould to explain the gaps in the fossil record - the fact that the fossil record does not show smooth evolutionary transitions. -

Predictability of a Large Intragenic Fitness Landscape
On the (un)predictability of a large intragenic fitness landscape Claudia Banka,b,c,1, Sebastian Matuszewskib,c,1, Ryan T. Hietpasd,e, and Jeffrey D. Jensenb,c,2,3 aInstituto Gulbenkian de Ciencia,ˆ 2780-156 Oeiras, Portugal; bSchool of Life Sciences, Ecole Polytechnique Fed´ erale´ de Lausanne, 1015 Lausanne, Switzerland; cSwiss Institute of Bioinformatics, 1015 Lausanne, Switzerland; dEli Lilly and Company, Indianapolis, IN 46225; and eDepartment of Biochemistry & Molecular Pharmacology, University of Massachusetts Medical School, Worcester, MA 01605 Edited by Andrew G. Clark, Cornell University, Ithaca, NY, and approved October 11, 2016 (received for review August 2, 2016) The study of fitness landscapes, which aims at mapping geno- of previously observed beneficial mutations or on the dissection types to fitness, is receiving ever-increasing attention. Novel exper- of an observed adaptive walk (i.e., a combination of muta- imental approaches combined with next-generation sequencing tions that have been observed to be beneficial in concert). (NGS) methods enable accurate and extensive studies of the fitness Secondly, theoretical research has proposed different land- effects of mutations, allowing us to test theoretical predictions scape architectures [such as the House-of-Cards (HoC), the and improve our understanding of the shape of the true under- Kauffman NK (NK), and the Rough Mount Fuji (RMF) model], lying fitness landscape and its implications for the predictability studied their respective properties, and developed a number and repeatability of evolution. Here, we present a uniquely large of statistics that characterize the landscape and quantify the multiallelic fitness landscape comprising 640 engineered mutants expected degree of epistasis (i.e., interaction effects between that represent all possible combinations of 13 amino acid-changing mutations) (10–14). -

EXPERIMENTAL EVIDENCE for SALTATIONAL CHROMOSOME EVOLUTION in CAL YCADENIA PAUCIFLORA GRAY (ASTERACEAE) Pauciflora Are Fairly Fe
Heredity (1980), 45 (1), 107-112 0018-067X/80/01940107$02.0O 1980. The Genetical Society of Great Britain EXPERIMENTALEVIDENCE FOR SALTATIONAL CHROMOSOME EVOLUTION IN CAL YCADENIA PAUCIFLORA GRAY (ASTERACEAE) GERALD D. CARR Department of Botany, University of Hawaii, 3190 Maile Way, Honolulu, Hawaii 96822, U.S.A. Received14.i.80 SUMMARY The fertility of the F1 structural heterozygote formed by crossing two aneuploid chromosome races of Calycadenia pauciflora is high despite the fact they are differentiated by the equivalent of three chromosome translocations. This and the fact that ancestral and derived structural homozygotes were recovered in experimental F2 and F3 progenies support the hypothesis that the derived race could have originated directly from the ancestral race in nature through a single saltational event involving multiple chromosome breaks. Two individuals with structurally unique, recombined chromosomes were also recovered in the F2 and the evolutionary potential of such products of meiosis in structural heterozygotes is considered to be significant. 1. INTRODUCTION THERE have been several experimental studies documenting the existence of pairs or groups of diploid plant species differentiated by little more than chromosome alterations, often accompanied by aneuploidy. Calycadenia, Chaenactis, Clarkia and Crepis are just a few of the genera in which this situation is best known or more frequent. In Clarkia, Lewis (1966) referred to this process of differentiation as saltational speciation. Others have used the term quantum evolution or quantum speciation in reference to this general phenomenon (cf. Grant, 1971). This type of chromosomal evolution requires that a new structural heterozygote pass through a "bottleneck of sterility" before the derived structural homozygote can be produced (cf. -

Myths and Legends of the Baldwin Effect
Myths and Legends of the Baldwin Effect Peter Turney Institute for Information Technology National Research Council Canada Ottawa, Ontario, Canada, K1A 0R6 [email protected] Abstract ary computation when there is an evolving population of learning individuals (Ackley and Littman, 1991; Belew, This position paper argues that the Baldwin effect 1989; Belew et al., 1991; French and Messinger, 1994; is widely misunderstood by the evolutionary Hart, 1994; Hart and Belew, 1996; Hightower et al., 1996; computation community. The misunderstandings Whitley and Gruau, 1993; Whitley et al., 1994). This syn- appear to fall into two general categories. Firstly, ergetic effect is usually called the Baldwin effect. This has it is commonly believed that the Baldwin effect is produced the misleading impression that there is nothing concerned with the synergy that results when more to the Baldwin effect than synergy. A myth or legend there is an evolving population of learning indi- has arisen that the Baldwin effect is simply a special viduals. This is only half of the story. The full instance of synergy. One of the goals of this paper is to story is more complicated and more interesting. dispel this myth. The Baldwin effect is concerned with the costs Roughly speaking (we will be more precise later), the and benefits of lifetime learning by individuals in Baldwin effect has two aspects. First, lifetime learning in an evolving population. Several researchers have individuals can, in some situations, accelerate evolution. focussed exclusively on the benefits, but there is Second, learning is expensive. Therefore, in relatively sta- much to be gained from attention to the costs. -

Tempo and Mode in the Macroevolutionary Reconstruction of Darwinism STEPHEN JAY GOULD Museum of Comparative Zoology, Harvard University, Cambridge, MA 02138
Proc. Nadl. Acad. Sci. USA Vol. 91, pp. 6764-6771, July 1994 Colloquium Paper This paper was presented at a coloquium ented "Tempo and Mode in Evolution" organized by Walter M. Fitch and Francisco J. Ayala, held January 27-29, 1994, by the National Academy of Sciences, in Irvine, CA. Tempo and mode in the macroevolutionary reconstruction of Darwinism STEPHEN JAY GOULD Museum of Comparative Zoology, Harvard University, Cambridge, MA 02138 ABSTRACT Among the several central nings of Dar- But conceptual complexity is not reducible to a formula or winism, his version ofLyellian uniformitranism-the extrap- epigram (as we taxonomists of life's diversity should know olationist commitment to viewing causes ofsmall-scale, observ- better than most). Too much ink has been wasted in vain able change in modern populations as the complete source, by attempts to define the essence ofDarwin's ideas, or Darwin- smooth extension through geological time, of all magnitudes ism itself. Mayr (1) has correctly emphasized that many and sequences in evolution-has most contributed to the causal different, if related, Darwinisms exist, both in the thought of hegemony of microevolutlon and the assumption that paleon- the eponym himself, and in the subsequent history of evo- tology can document the contingent history of life but cannot lutionary biology-ranging from natural selection, to genea- act as a domain of novel evolutionary theory. G. G. Simpson logical connection of all living beings, to gradualism of tried to combat this view of paleontology as theoretically inert change. in his classic work, Tempo and Mode in Evolution (1944), with It would therefore be fatuous to claim that any one legit- a brilliant argument that the two subjects of his tide fall into a imate "essence" can be more basic or important than an- unue paleontological domain and that modes (processes and other. -

Punctuated Equilibrium Models in Organizational Decision Making 135
1 2 Punctuated Equilibrium Models 3 4 8 5 in Organizational Decision 6 7 Making 8 9 10 Scott E. Robinson 11 12 13 14 CONTENTS 15 16 8.1 Two Research Conundrums..................................................................................................134 17 8.1.1 Lindblom’s Theory of Administrative Incrementalism...........................................134 18 8.1.2 Wildavsky’s Theory of Budgetary Incrementalism.................................................135 19 8.1.3 The Diverse Meanings of Budgetary “Incrementalism”..........................................135 20 8.1.4 A Brief Aside on Paleontology.................................................................................136 21 8.2 Punctuated Equilibrium Theory—A Way Out of Both Conundrums.................................136 22 8.3 A Theoretical Model of Punctuated Equilibrium Theory....................................................137 23 8.4 Evidence of Punctuated Equilibria in Organizational Decision Making ............................139 24 8.4.1 Punctuated Equilibrium and the Federal Budget.....................................................139 25 8.4.2 Punctuated Equilibrium and Local Government Budgets.......................................140 26 8.4.3 Punctuated Equilibrium and the Federal Policy Process.........................................141 27 8.4.4 Punctuated Equilibrium and Organizational Bureaucratization ..............................142 28 8.4.5 Assessing the Evidence.............................................................................................143 -

Darwinian Evolution and Quantum Evolution
tics: Cu ne rr e en Nemer et al., Hereditary Genet 2017, 6:2 G t y R r e a t s i e DOI: 10.4172/2161-1041.1000181 d a e r r c e h H Hereditary Genetics ISSN: 2161-1041 Research Article Open Access Darwinian Evolution and Quantum Evolution are Complementary: A Perspective Georges Nemer1, Christina Bergqvist2 and Mazen Kurban1,2,3* 1Department of Biochemistry and Molecular Genetics, American University of Beirut, Beirut, Lebanon 2Department of Dermatology, American University of Beirut, Beirut, Lebanon 3Department of Dermatology, Columbia University, New York, USA Abstract Evolutionary biology has fascinated scientists since Charles Darwin who cornered the concept of natural selection in the 19th century. Accordingly, organisms better adapted to their environment tend to survive and produce more offspring; in other terms, randomly occurring mutations that render the organism more fit to survival will be carried on and be transmitted to the offspring. Nearly a century later, science has seen the discovery of quantum mechanics, the branch of mechanics that deals with subatomic particles. Along with it, came the theory of quantum evolution whereby quantum effects can bias the process of mutation towards providing an advantage for organism survival. This is consistent with looking at the biological system as being a product of chemical-physical reactions, such that chemical structures arrange according to physical laws to form a replicative material referred to as the DNA. In this report, we attempt to reconcile both theories, trying to demonstrate that they complement each other, hoping to fill the gaps in our understandings of the versatility of the mutational status of the DNA as an essential mechanism of life compatibility. -
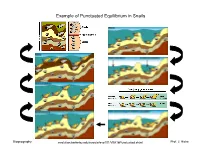
Example of Punctuated Equilibrium in Snails
Example of Punctuated Equilibrium in Snails Biogeography evolution.berkeley.edu/evosite/evo101/VIIA1bPunctuated.shtml1 Prof. J. Hicke Punctuated Equilibrium Lomolino et al. , 2006 Biogeography 2 Prof. J. Hicke Allopatric Speciation http://wps.pearsoncustom.com/wps/media/objects/3014/3087289/Web_Tutorials/18_A01.swf Biogeography 3 Prof. J. Hicke Allopatric Speciation: Vicariance Event Biogeography 4 Prof. J. Hicke Allopatric speciation, founder event Genes rare in original population are dominant in founding population Biogeography 5 Prof. J. Hicke Sympatric and Parapatric Speciation sympatric: extensive overlap parapatric: minimal overlap (partial geographic separation) Lomolino et al. , 2006 Biogeography 6 Prof. J. Hicke Parapatric Speciation No extrinsic barrier to gene flow, but… 1. restricted gene flow within population 2. varying selective pressures across the population range “Although continuously distributed, different flowering times have begun to reduce gene flow between metal-tolerant plants and metal-intolerant plants. “ evolution.berkeley.edu/evosite/evo101/VC1dParapatric.shtml Biogeography 7 Prof. J. Hicke Example of Sympatric Speciation • 200 years ago, flies only on hawthorns • then, introduction of domestic apple • females lay eggs on type of fruit they grew up on; males look for mates on type of fruit they grew up on • restricted gene flow • speciation http://evolution.berkeley.edu/evosite/evo101/VC1eSympatric.shtml Biogeography 8 Prof. J. Hicke Example of Sympatric Speciation Lomolino et al. , 2006 Biogeography 9 Prof. J. Hicke Adaptive Radiation often rapid speciation: Lake Victoria: 100s of new species in <12,000 years Lomolino et al. , 2006 Biogeography 10 Prof. J. Hicke Adaptive Radiation www.micro.utexas.edu/courses/levin/bio304/evolution/speciation.html Biogeography 11 Prof. -

Quantum Microbiology
Curr. Issues Mol. Biol. 13: 43-50. OnlineQuantum journal at http://www.cimb.orgMicrobiology 43 Quantum Microbiology J. T. Trevors1* and L. Masson2* big bang. Currently, one of the signifcant unsolved problems in modern physics is how to merge the two into a unifying 1School of Environmental Sciences, University of Guelph, theory. Since quantum mechanics describes the physical 50 Stone Rd., East, Guelph, Ontario N1G 2W1, Canada world, and living organisms are physical entities, it is rational 2Biotechnology Research Institute, National Research and logical to examine the role of quantum mechanics in Council of Canada, 6100 Royalmount Ave., Montreal, the matter and energy of living microorganisms, especially Quebec H4P 2R2, Canada their origin about 4 billion years ago. To do so requires an understanding of quantum processes at the atomic scale and smaller where electrons, for example, do not collide Abstract with the atomic nucleus but defy electromagnetism and orbit During his famous 1943 lecture series at Trinity College at both an undefned speed and path around the nucleus. Dublin, the reknown physicist Erwin Schrödinger discussed One distinguishing characteristic of quantum mechanics the failure and challenges of interpreting life by classical is the Complementarity Principle (or wave-particle duality) physics alone and that a new approach, rooted in Quantum developed by Niels Bohr indicating that a particle can principles, must be involved. Quantum events are simply a possess multiple contradictory properties. level of organization below the molecular level. This includes A classic example of complementarity is Thomas the atomic and subatomic makeup of matter in microbial Young's famous light interference, and later on the double- metabolism and structures, as well as the organic, genetic slit, experiment showing that light or other quantum information codes of DNA and RNA.