From Dominican Amber
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Household Insects of the Rocky Mountain States
Household Insects of the Rocky Mountain States Bulletin 557A January 1994 Colorado State University, University of Wyoming, Montana State University Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, Milan Rewerts, interim director of Cooperative Extension, Colorado State University, Fort Collins, Colorado. Cooperative Extension programs are available to all without discrimination. No endorsement of products named is intended nor is criticism implied of products not mentioned. FOREWORD This publication provides information on the identification, general biology and management of insects associated with homes in the Rocky Mountain/High Plains region. Records from Colorado, Wyoming and Montana were used as primary reference for the species to include. Mention of more specific localities (e.g., extreme southwestern Colorado, Front Range) is provided when the insects show more restricted distribution. Line drawings are provided to assist in identification. In addition, there are several lists based on habits (e.g., flying), size, and distribution in the home. These are found in tables and appendices throughout this manual. Control strategies are the choice of the home dweller. Often simple practices can be effective, once the biology and habits of the insect are understood. Many of the insects found in homes are merely casual invaders that do not reproduce nor pose a threat to humans, stored food or furnishings. These may often originate from conditions that exist outside the dwelling. Other insects found in homes may be controlled by sanitation and household maintenance, such as altering potential breeding areas (e.g., leaky faucets, spilled food, effective screening). -

From Guatemala
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 2019 A contribution to the knowledge of Dermestidae (Coleoptera) from Guatemala José Francisco García Ochaeta Jiří Háva Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Ecology and Evolutionary Biology Commons, and the Entomology Commons This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. December 23 2019 INSECTA 5 ######## A Journal of World Insect Systematics MUNDI 0743 A contribution to the knowledge of Dermestidae (Coleoptera) from Guatemala José Francisco García-Ochaeta Laboratorio de Diagnóstico Fitosanitario Ministerio de Agricultura Ganadería y Alimentación Petén, Guatemala Jiří Háva Daugavpils University, Institute of Life Sciences and Technology, Department of Biosystematics, Vienības Str. 13 Daugavpils, LV - 5401, Latvia Date of issue: December 23, 2019 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL José Francisco García-Ochaeta and Jiří Háva A contribution to the knowledge of Dermestidae (Coleoptera) from Guatemala Insecta Mundi 0743: 1–5 ZooBank Registered: urn:lsid:zoobank.org:pub:10DBA1DD-B82C-4001-80CD-B16AAF9C98CA Published in 2019 by Center for Systematic Entomology, Inc. P.O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non- marine arthropod. Topics considered for publication include systematics, taxonomy, nomenclature, checklists, faunal works, and natural history. -

16 Endemic Insect Species from the Algodones Sand Dunes, Imperial County, California As Federally Endangered Or Threatened Under the Federal Endangered Species Act
July 19, 2004 Ms. Gale Norton Secretary of the Interior Department of the Interior 1849 C Street, N.W. Washington, D. D. 20240 Fax: (202) 208-6956 Mr. Jim Bartel Field Supervisor Carlsbad Fish and Wildlife Office 6010 Hidden Valley Road Carlsbad, CA 92009 Fax: (760) 431-9624 Dear Ms. Norton and Mr. Bartel, Enclosed please find a petition to list 16 insect species endemic to the Algodones Dunes, Imperial County, California as threatened or endangered pursuant to the Endangered Species Act, 16 U.S.C. 1531 et seq. The petition is submitted by the Center for Biological Diversity, Public Employees for Environmental Responsibility, and the Sierra Club. Petitioners will be sending supporting documentation in a follow-up mailing. Thank you for your consideration of this petition. Sincerely, Monica L. Bond Center for Biological Diversity Karen Schambach Public Employees for Environmental Responsibility George Barnes Sierra Club Petition to List 16 Endemic Insect Species from the Algodones Sand Dunes, Imperial County, California as Federally Endangered or Threatened under the Federal Endangered Species Act Photo by Andrew Harvey The Center for Biological Diversity hereby formally petitions to list: two sand wasps (Microbembex elegans Griswold and Stictiella villegasi Bohart); two bees (Perdita algodones Timberlake and P. glamis Timberlake); one vespid (Euparagia n. sp.); two velvet ants (Dasymutilla nocturna Mickel and Dasymutilla imperialis Manley and Pitts); three jewel beetles (Algodones sand jewel beetle, Lepismadora algodones Velten, Algodones white wax jewel beetle, Prasinalia imperialis (Barr), and Algodones Croton jewel beetle, Agrilus harenus Nelson); two scarab beetles (Hardy’s dune beetle, Anomala hardyorum Potts and Cyclocephala wandae); and four subspecies of Roth’s dune weevil (Trigonoscuta rothi rothi, T. -

Bibliography
Bibliography - A - Abeille de Perrin E. 1870: Megatoma rufovittata sp. nov. Annales de la Société Entomologique de France 10: 46. Abeille de Perrin E. 1872: Études sur les Coléoptères cavernicoles, suivies de la description de Coléoptères nouveaux propres au midi de la France. Annales de la Société Entomologique de France 1872: 41-44. Abdel-Kawy F. K. 1998: Effect of gamma-irradition on some biological activities of the larval stage of the khapra beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae). Journal Egyptian German Society Zoology 27: 141-151. Abdel-Kawy F. K. 1999: Effect of gamma-irradiation on some biological activities of the larval stage of the khapra beetle, Trogoderma granarium Everts (Col., Dermestidae). Journal of Applied Entomology 123: 201-204. Abdel-Kedar M. M. & Barak A. V. 1979: Evidence for a sex pheromone in the hide beetle, Dermestes maculatus (DeGeer) (Coleoptera: Dermestidae). Journal of Chemical Ecology 5: 805-813. Abdel-Rahman H. A., Zenab A. Soliman & Ali M. F. 1981a: Biological study on the black carpet beetle Attagenus scalaris Pic (Coleoptera: Dermestidae). Bulletin of the Society of Egypte 63: 231-241. Abdel-Rahman H. A., Zenab A. Soliman & Ali M. F. 1981b: Ecological studies on the black carpet beetle Attagenus scalaris Pic (Coleoptera: Dermestidae). Bulletin of the Society of Egypte 63: 243-252. Abivardi C. 2001: Iranian Entomology, An Introduction. Volume 2: Applied Entomology. Schriftenreihe der Stiftung Franz Xaver Schnyder von Wartensee, Zentralbibliothek Zürich 59: 445-1033. Ádám L. 1986: The species of Elateroidea, Dryopoidea, Byrrhoidea, Dermestoidea and Bostrychoidea of the Kiskunság (Coleoptera). In.: Mahunka S. (ed.): The Fauna of Kishunság National Park I. -

A New U.S. and Florida Record for Caccoleptus (Bicaccoleptus) Kacka Háva, 2009 (Coleoptera: Dermestidae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 6-15-2012 A new U.S. and Florida record for Caccoleptus (Bicaccoleptus) kacka Háva, 2009 (Coleoptera: Dermestidae) Jiří Háva Prague-west, Czech Republic, [email protected] Michael C. Thomas Florida State Collection of Arthropods, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Háva, Jiří and Thomas, Michael C., "A new U.S. and Florida record for Caccoleptus (Bicaccoleptus) kacka Háva, 2009 (Coleoptera: Dermestidae)" (2012). Insecta Mundi. 750. https://digitalcommons.unl.edu/insectamundi/750 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA A Journal of World Insect Systematics MUNDI 0237 A new U.S. and Florida record for Caccoleptus (Bicaccoleptus) kacka Háva, 2009 (Coleoptera: Dermestidae) Jiří Háva Private Entomological Laboratory and Collection Rýznerova 37/37, CZ - 252 62, Únětice u Prahy Prague-west, Czech Republic [email protected] Michael C. Thomas Florida State Collection of Arthropods Florida Department of Agriculture & Consumer Services P.O. Box 147100 Gainesville, FL 32614-7100, USA [email protected] Date of Issue: June 15, 2012 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Jiří Háva and Michael Thomas A new U.S. and Florida record for Caccoleptus (Bicaccoleptus) kacka Háva, 2009 (Co- leoptera: Dermestidae) Insecta Mundi 0237: 1–3 Published in 2012 by Center for Systematic Entomology, Inc. -

1469 Hava.Vp
A new genus and species of Dermestidae (Coleoptera) from the Eckfeld Maar crater (Middle Eocene, Germany) JIØÍ HÁVA & TORSTEN WAPPLER Eckfeldattagenus eocenicus gen. et sp. nov. is described from the Middle Eocene Eckfeld Maar (Germany) on the basis of completely preserved specimens, representing one of the rare reports of fossil skin beetles (Coleoptera: Dermestidae: Attageninae). The new genus and species differs from all other Dermestidae by the very flat body form and pronotum, and its unique structure of the antennal club and the wrinkled elytral surface. • Key words: taxonomy, new genus, new species, fossil, Coleoptera, Dermestidae, Germany. HÁVA,J.&WAPPLER, T. 2014. A new genus and species of Dermestidae (Coleoptera) from the Eckfeld Maar crater (Middle Eocene, Germany). Bulletin of Geosciences 89(1), 67–74 (1 figure, appendix). Czech Geological Survey, Prague. ISSN 1214-1119. Manuscript received July 24, 2013; accepted in revised form October 23, 2013; published on- line December 12, 2013; issued January 21, 2014. Jiří Háva, Department of Forest Protection and Entomology, Faculty of Forestry and Wood Sciences, Czech University of Life Sciences, Kamýcká 1176, CZ-165 21, Prague 6 – Suchdol, Czech Republic • Torsten Wappler (corresponding au- thor), Steinmann Institut für Geologie, Mineralogie und Paläontologie, Universität Bonn, Nussallee 8, 53115 Bonn, Germany; [email protected] Dermestidae commonly referred to as skin, larder and car- from the Triassic deposits of Queensland, described by Dun- pet beetles are characterized by a variety of ecological ha- stan (1923), but the assignment of these fossils was doubted bits and a fascinating biology. Most genera are scavengers by Háva & Prokop (2004). -

Coleoptera) from Australia - Part 5
Studies and Reports Taxonomical Series 15 (2): 323-328, 2019 A contribution to knowledge of Dermestidae (Coleoptera) from Australia - Part 5. Subfamily Trinodinae from Queensland and New Caledonia Jiří HÁVA1,2 1Daugavpils University, Institute of Life Sciences and Technology, Department of Biosystematics, Vienības Str. 13, Daugavpils, LV - 5401, Latvia 2Private Entomological Laboratory and Collection, Rýznerova 37, CZ - 252 62 Únětice u Prahy, Praha-západ, Czech Republic e-mail: [email protected] Taxonomy, new species, new records, Coleoptera, Dermestidae, Trinodinae, Evorinea, Trichelodes, Trinoparvus, Australia, New Caledonia Abstract. The following new species are described, illustrated and compared with similar taxa: Trichelodes caledonicus sp. nov. (New Caledonia) and Trinoparvus suturalis sp. nov. (Australia: Queensland). The species Evorinea iota (Arrow, 1915) is newly recorded from Australia: Queensland. A key to Australian species belonging to Trinodinae is provided. INTRODUCTION The subfamily Trinodinae Casey, 1900 recently contains 10 genera classed in 4 tribes worldwide (Háva 2015). Only an introduced species Thylodrias contractus Motschulsky, 1839 and an endemic species Trichelodes delicatula Carter, 1935 have still been known. In the present article, a further species is newly recorded for Australia and two species are newly described from Australia: Queensland and New Caledonia. The article follows preceding articles about Dermestidae published by the present author (Háva 2016, 2017, 2018, Háva & Horák 2017). MATERIAL AND METHODS The size of the beetles or of their body parts can be useful in species recognition and thus, the following measurements were made: total length (TL) - linear distance from anterior margin of pronotum to apex of elytra. elytral width (EW) - maximum linear transverse distance. -

Occurrence, Ecological Function and Medical Importance of Dermestid Beetle Hastisetae
Occurrence, ecological function and medical importance of dermestid beetle hastisetae Enrico Ruzzier1, Marcin Kadej2 and Andrea Battisti1 1 Department of Agronomy, Food, Natural Resources, Animals and the Environment (DAFNAE), Università degli Studi di Padova, Padova, Italy 2 Department of Invertebrate Biology, Evolution and Conservation, University of Wrocªaw, Wrocªaw, Poland ABSTRACT Hastisetae are a specific group of detachable setae characterizing the larvae of Megatom- inae (Coleoptera: Dermestidae), commonly known as carpet and khapra beetles. These setae are located on both thoracic and abdominal tergites and they are the primary defense of the larva against invertebrate predators. According to previous studies, the main purpose of hastisetae is to work as a mechanical obstacle, but they are also capable to block and kill a predator. Hastisetae, single or aggregate, function as an extremely efficient mechanical trap, based on an entangling mechanism of cuticular structures (spines and hairs) and body appendages (antennae, legs and mouthparts). It is believed that this defensive system evolved primarily to contrast predation by invertebrates, however it has been observed that hastisetae may affect vertebrates as well. Although information on the impacts of vertebrate predators of the beetles is lacking, hastisetae have been shown to be a possible threat for human health as an important contaminant of stored products (food and fabric), work and living environment. Review of past and recent literature on dermestid larvae has revealed that despite these structures indicated as one of the distinctive characters in species identification, very little is known about their ultrastructure, evolution and mechanism of action. In the present work, we will provide the state of knowledge on hastisetae in Dermestidae and we will present and Submitted 1 July 2019 discuss future research perspectives intended to bridge the existing knowledge gaps. -

Checklist of Dermestidae (Insecta: Coleoptera: Bostrichoidea) of the United States
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 6-25-2021 Checklist of Dermestidae (Insecta: Coleoptera: Bostrichoidea) of the United States Jiří Háva Andreas Herrmann Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Ecology and Evolutionary Biology Commons, and the Entomology Commons This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. A journal of world insect systematics INSECTA MUNDI 0871 Checklist of Dermestidae (Insecta: Coleoptera: Bostrichoidea) Page Count: 16 of the United States Jiří Háva Author et al. Forestry and Game Management Research Institute Strnady 136, CZ-156 00 Praha 5 - Zbraslav, Czech Republic Andreas Herrmann Bremervörder Strasse 123, 21682 Stade, Germany Date of issue: June 25, 2021 Center for Systematic Entomology, Inc., Gainesville, FL Háva J, Herrmann A. 2021. Checklist of Dermestidae (Insecta: Coleoptera: Bostrichoidea) of the United States. Insecta Mundi 0871: 1–16. Published on June 25, 2021 by Center for Systematic Entomology, Inc. P.O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non- marine arthropod. Topics considered for publication include systematics, taxonomy, nomenclature, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medi- cal entomology, pest control research, etc.), and no longer publishes book reviews or editorials. -
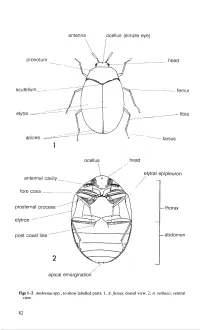
Vol 5 Part 3. Adults and Larvae of Hide, Larder and Carpet Beetles and Their Relatives
antenna ocellus (simple eye) I pronotum ____ head --------- - -- femur apices 1 ocellus head elytral epipleuron antenna! cavity __ / presternal process thorax elytron - --------------- post coxal line ------- abdomen 2 / / apical emargination Figs 1-2. Anthrenus spp., to show labelled parts. I, A.fuscus, dorsal view. 2, A. verbasci, ventral view. 82 coxa basal antenna! segment presternum ~head \\ \ apical antenna! segment hypomeron ~ I I antenna! club ......... elytral epipleuron / mesosternum coxal plate ~ trochanter 1 2 setal tuft / J I lateral impressed line tarsus / I I \ elytron visible abdominal sternite 3 Fig. 3. Ventral view of Dermestes sp., to show labelled parts. 83 Figs 4--7. 4- 5, Thylodrias contractus. 4, male. 5, female. 6, Trinodes sp., after Hinton (1945). 7, Thorictodes heydeni. Scale lines = 1.0 mm. 84 metepisternum I metasternum -~---a_b_d_o_m_en ---------- 8 hind coxa ocellus compound eye mouth parts 9 fore coxa 10 Figs 8-10. 8, diagram to show position of hind coxae of Trinodes hirtus. 9- 10, side view of head and prothorax (appendages removed). 9, Attagenus brunneus. 10, Megatoma undata. 85 [ 11 12 13 14 Figs 11-14. Ventral view of adult. 11 , Dermestes peruvianus. 12, Attagenus unicolor. 13, Trogoderma inc/usum. 14, Reesa vespulae. Scale lines = 1.0 mm. 86 Figs 15--20. Ventral view of adult. 15, Globicornis nigripes. 16, Megatoma unda ta. 17, Ctesias serra. 18, Anthrenus fuscus. 19, Anthrenocerus australis. 20, Trinodes hirtus. Scale lines = 1.0 mm. 87 Figs 21-24. Dermestes spp. 21, D. macu/atus. 22, D. frischii. 23, D. carnivorus. 24, D. ater. Scale line = 2.0 mm. All after Hinton (1945). -

New Faunistic Records and Remarks on Dermestidae (Coleoptera) - Part 19
Natura Somogyiensis 33: 27-36. Ka pos vár, 2019 DOI:10.24394/NatSom.2019.33.27 Submitted: 05.05, 2019; Accepted: 10.05.2019; Published: 31.05.2019. www.smmi.hu/termtud/ns/ns.htm New faunistic records and remarks on Dermestidae (Coleoptera) - Part 19. Jiří Háva1,2 & Andreas Herrmann3 1Daugavpils University, Institute of Life Sciences and Technology, Department of Biosystematics, Vienības Str. 13, Daugavpils, LV - 5401, Latvia 2Private Entomological Laboratory and Collection, Rýznerova 37, CZ - 252 62 Únětice u Prahy, Praha-západ, Czech Republic, e-mail: [email protected] 3Bremervörder Strasse 123, D-21682, Stade, Germany e-mail: [email protected] Háva, J. & Hermmann, A.: New faunistic records and remarks on Dermestidae (Coleoptera) - Part 19. Abstract: The corrections to the Holloway´s articles about Anthrenus species from Mallorca are provided. The species Anthrenus (s. str.) amandae Holloway, 2019 syn. nov., is a new junior synonym of Anthrenus (s. str.) pimpinellae (Fabricius, 1775). The corrections to Pushkin´s article about Dermestidae are discussed. The fol- lowing species are newly recorded: Anthrenus (Anthrenops) coloratus Reitter, 1881 (U.S.A.: Delaware); Anthrenus (Anthrenus) mroczkowskii Kalík, 1954 (Macedonia); Anthrenus (Anthrenus) munroi Hinton, 1943 (Greece: Crete); Anthrenus (Anthrenus) picturatus picturatus Solsky, 1876 (Romania); Anthrenus (Anthrenus) pimpinellae isabellinus Küster, 1848 (U.S.A.: Delaware); Anthrenus (Anthrenus) scrophulariae (Linnaeus, 1758) (U.S.A.: Delaware); Anthrenus (Helocerus) fuscus -

(Coleoptera: Bostrichiformia) from the Philippines
Acta Biol. Univ. Daugavp. 16 (2) 2016 ISSN 1407 - 8953 A CONTRIBUTION TO KNOWLEDGE OF DERMESTIDAE (COLEOPTERA: BOSTRICHIFORMIA) FROM THE PHILIPPINES Jiří Háva Háva J. 2016. A contribution to knowledge of Dermestidae (Coleoptera: Bostrichiformia) from the Philippines. Acta Biol. Univ. Daugavp., 16 (2): 129 – 134. Two new species, Attagenus (Aethriostoma) philippinensis sp. nov. and Anthrenus (Nathrenus) hrdlickai sp. nov., from the Philippines are described, illustrated and compared with similar species. A new synonymy is proposed: Thumaglossa dembyckyi Háva, 2002 (= Thaumaglossa bellissima Háva, 2005 syn. nov.). A list of all species recorded from the Philippines is proposed. Key words: Coleoptera, Dermestidae, taxonomy, new species, faunistics, the Philippines. Jiří Háva, Department of Forest Protection and Entomology,Faculty of Forestry and Wood Sciences,Czech University of Life Sciences,Kamýcká 1176, CZ-165 21, Prague 6 - Suchdol, Czech Republic, E-mail: [email protected] INTRODUCTION anterior margin of pronotum to apex of elytra. The family Dermestidae currently consists of 62 • elytral width (EW) - maximum linear genera, containing ca. 1600 species worldwide transverse distance. (Háva 2015); 29 species are known from the Philippines (Mroczkowski 1968, Caliboso et al. Deposition of type material: JHAC - Jiří Háva, 1986, Háva 2005, 2012, 2015a,b, 2016, Háva et Private Entomological Laboratory & Collection, al. 2016). Únětice u Prahy, Prague-West, Czech Republic. The nomenclature and zoogeography follow In the present article, results of studying material Háva (2015a). recently collected on the Philippines, isles Mindanao and Luzon are presented. Two new The all colour photographs taken by a Nikon species are described and a new synonym is Coolpix 990 digital camera through an MBS-10 proposed.