IBM Watson Health in Oncology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Artificial Intelligence in Health Care: the Hope, the Hype, the Promise, the Peril
Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril Michael Matheny, Sonoo Thadaney Israni, Mahnoor Ahmed, and Danielle Whicher, Editors WASHINGTON, DC NAM.EDU PREPUBLICATION COPY - Uncorrected Proofs NATIONAL ACADEMY OF MEDICINE • 500 Fifth Street, NW • WASHINGTON, DC 20001 NOTICE: This publication has undergone peer review according to procedures established by the National Academy of Medicine (NAM). Publication by the NAM worthy of public attention, but does not constitute endorsement of conclusions and recommendationssignifies that it is the by productthe NAM. of The a carefully views presented considered in processthis publication and is a contributionare those of individual contributors and do not represent formal consensus positions of the authors’ organizations; the NAM; or the National Academies of Sciences, Engineering, and Medicine. Library of Congress Cataloging-in-Publication Data to Come Copyright 2019 by the National Academy of Sciences. All rights reserved. Printed in the United States of America. Suggested citation: Matheny, M., S. Thadaney Israni, M. Ahmed, and D. Whicher, Editors. 2019. Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril. NAM Special Publication. Washington, DC: National Academy of Medicine. PREPUBLICATION COPY - Uncorrected Proofs “Knowing is not enough; we must apply. Willing is not enough; we must do.” --GOETHE PREPUBLICATION COPY - Uncorrected Proofs ABOUT THE NATIONAL ACADEMY OF MEDICINE The National Academy of Medicine is one of three Academies constituting the Nation- al Academies of Sciences, Engineering, and Medicine (the National Academies). The Na- tional Academies provide independent, objective analysis and advice to the nation and conduct other activities to solve complex problems and inform public policy decisions. -

Integration of System for Research in Health with Computation in Cloud Using Artificial Intelligence
MOJ Proteomics & Bioinformatics Research Article Open Access Integration of system for research in health with computation in cloud using artificial intelligence Abstract Volume 7 Issue 4 - 2018 Currently the use of information technology is often applied in the health area. With regard 1 to scientific research, we have the SINPE© Integrated System of Electronic Protocols. Carlos Henrique Kuretzki, José Simão de 2 3 The purpose of this tool is to support the researcher in this area who until now did not Paula Pinto, José Claudio Vianna have statistical tests. The main objective of this work is to provide SINPE© users with the 1Professor of the Program for Analysis and Development of improvement in health data analysis through the use of cognitive computing technologies. Systems and Computer Engineering, Federal University, Brazil 2Professor of the Information Management Program, Federal Keywords: artificial intelligence, cloud computing, information systems University, Brazil 3Professor of the Computer Engineering, Universidade Positivo, Brazil Correspondence: Carlos Henrique Kuretzki, Professor of the Program for Analysis and Development of Systems and Computer Engineering, Federal University, Brazil, Email [email protected] Received: June 29, 2018 | Published: August 08, 2018 Introduction more quickly.4 IBM Watson is a cognitive computing technology and have been used to investigate the biological and health sciences. The health sector is one of the largest in most countries and can It is based on medical literature, patents, genetic, and chemical and significantly benefit from high‒quality, real‒time and location‒ pharmacological data.5 The IBM’s Watson platform combines some independent data. However, many healthcare professionals are capabilities, building a complete cognitive system, which is being not familiar with information technology solutions, business and called the new era of computing. -

Data Transfer Options and Requirements
Data Transfer Options and Requirements Original date 25 Jan 2005 Revision date 21 Aug 2018 Proprietary and Confidential Data Transfer Options and Requirements Summary of changes Date Short description of changes September 2016 Updated name of submission tool to SDSS. August 2018 Rebranded. Contacting support Support is available through the following link: https://www.ibm.com/software/support/watsonhealth/truven_support.html © Copyright IBM Corporation 2018 IBM Confidential 2 Data Transfer Options and Requirements Contents Summary of changes .................................................................................................................................... 2 Contacting support ........................................................................................................................... 2 Contents ........................................................................................................................................................ 3 Overview ....................................................................................................................................................... 5 Electronic data transfer methods ..................................................................................................... 5 Data suppliers ..................................................................................................................... 5 Data recipients ................................................................................................................... -

Treatment and Differential Diagnosis Insights for the Physician's
Treatment and differential diagnosis insights for the physician’s consideration in the moments that matter most The role of medical imaging in global health systems is literally fundamental. Like labs, medical images are used at one point or another in almost every high cost, high value episode of care. Echocardiograms, CT scans, mammograms, and x-rays, for example, “atlas” the body and help chart a course forward for a patient’s care team. Imaging precision has improved as a result of technological advancements and breakthroughs in related medical research. Those advancements also bring with them exponential growth in medical imaging data. The capabilities referenced throughout this document are in the research and development phase and are not available for any use, commercial or non-commercial. Any statements and claims related to the capabilities referenced are aspirational only. There were roughly 800 million multi-slice exams performed in the United States in 2015 alone. Those studies generated approximately 60 billion medical images. At those volumes, each of the roughly 31,000 radiologists in the U.S. would have to view an image every two seconds of every working day for an entire year in order to extract potentially life-saving information from a handful of images hidden in a sea of data. 31K 800MM 60B radiologists exams medical images What’s worse, medical images remain largely disconnected from the rest of the relevant data (lab results, patient-similar cases, medical research) inside medical records (and beyond them), making it difficult for physicians to place medical imaging in the context of patient histories that may unlock clues to previously unconsidered treatment pathways. -
IBM Pricing Index New.Xlsx
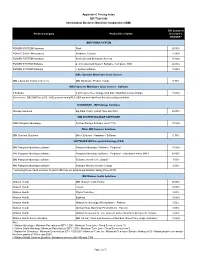
Appendix C Pricing Index DIR-TSO-3996 International Business Machines Corporation (IBM) DIR Customer Product Category Product Description Discount % off MSRP * IBM POWER SYSTEM POWER-SYSTEM Hardware Rack 26.00% POWER-SYSTEM Hardware Hardware Console 15.00% POWER-SYSTEM Hardware Scale Out and Enterprise Servers 15.00% POWER SYSTEM Software p: AIX, Linux and System Software, Compilers, HMC 26.00% POWER SYSTEM Software i: System software 18.00% IBM z Systems Mainframe Class Servers IBM z Systems Family of Servers IBM Mainframe Product Family 0.75% IBM Z Systems Mainframe Class Servers - Software z Software z OTC (One Time Charge) and MLC (Monthly License Charge 15.00% Exclusions - DB2 QMF for z/OS, VUE products and zIPLA S&S are excluded from this discounting schedule HARDWARE - IBM Storage Solutions Storage Solutions Big Data, Flash, Hybrid,Tape and SAN 25.00% IBM SYSTEM STOARGE SOFTWARE NON Passport Advantage System Storage Software not in PPA 15.00% Other IBM Systems Solutions IBM Systems Solutions Other Systems - Hardware / Software 0.75% SOFTWARE IBM Passport Advantage (PPA) IBM Passport Advantage software Passport Advantage Software - Perpetual 15.00% IBM Passport Advantage software Passport Advantage Software - Perpetual - Education Entities ONLY 60.00% IBM Passport Advantage software Software as a Service (SaaS)* 3.00% IBM Passport Advantage software Software Monthly License Charge 2.00% * excluding those SaaS products for which IBM has not established Relative Selling Price (RSP) IBM Watson Health Solutions Watson Health IBM Watson -

Watson Health Intro 170612
Empowering Heroes, Transforming Health Introduction to Watson Health IBM's statements regarding its plans, directions and intent are subject to change or withdrawal without notice at IBM's sole discretion. Information regarding potential future products is intended to outline our general product direction and it should not be relied on in making a purchasing decision. The information mentioned regarding potential future products is not a commitment, promise, or legal obligation to deliver any material, code or functionality. Information about potential future products may not be incorporated into any contract. The development, release, and timing of any future features or functionality described for our products remains at our sole discretion. Watson Health © IBM Corporation 2017 2 Time matters in healthcare, cognitive technologies, and machine learning. Watson Health © IBM Corporation 2017 3 IBM Leadership in Augmented Intelligence and Health: Over a Decade in Development 7,000 employees IBM enables an 10,000+ “evidence-based clients & partners Healthcare Memorial Sloan eco-system” Kettering Cancer Center Mayo Clinic Cognitive test Cleveland Clinic case results in creation of Watson 2005 2008 2010 2011 2012 2014 2014 2015 2016 Watson Health © IBM Corporation 2017 4 Global Shifts Drive Momentum R&D, Access, Delivery & Engagement Data Dynamic Value vs Efficient, Explosion Delivery Volume Effective R&D 150+ exabytes 50% $47 trillion 1 in 10 of healthcare Expected alternative payments Estimated global economic clinical Trials in cancer data today1 from Centers of Medicare & impact of chronic disease by are shut down from lack of Medicaid by 20184 20307 participation10 Over 230K 75%+ $3 trillion $2B active clinical trials2 of patients expected to use Estimated U.S. -

Senior Vice President, Cognitive Solutions and IBM Research IBM
Dr. John E. Kelly III Senior Vice President, Cognitive Solutions and IBM Research IBM As IBM senior vice president, Cognitive Solutions and IBM Research, Dr. John E. Kelly III is focused on the company’s investments in several of the fastest-growing and most strategic parts of the information technology market. His portfolio includes IBM Analytics, IBM Commerce, IBM Security and IBM Watson, as well as IBM Research and the company’s Intellectual Property team. He also oversees the development of units devoted to serving clients in specific industries, beginning with the 2015 launches of IBM Watson Health and IBM Watson Internet of Things. In this role, Dr. Kelly’s top priorities are to stimulate innovation in key areas of information technology and to bring those innovations into the marketplace quickly; to apply these innovations to help IBM clients succeed; and to identify and nurture new and future areas for investment and growth. He works closely with the leaders of these units to drive business development, accelerate technology transfer and invest for the future. Dr. Kelly was most recently senior vice president and director of IBM Research, only the tenth person to hold that position over the past seven decades. Under Dr. Kelly, IBM Research expanded its global footprint by adding four new labs (including IBM’s first in Africa, South America and Australia), creating a network of approximately 3,000 scientists and technical employees across 12 laboratories in 10 countries. During his tenure, IBM maintained and extended what is now 23 straight years of patent leadership. Most notably, Dr. -

Access a Patient's Complete Medical History When It Matters Most
Data Sheet Access a patient’s complete medical history when it matters most IBM iConnect Enterprise Archive Today, it is common for health care to be delivered across multiple settings. A patient’s diagnosis and treatment journey can rapidly take them from a physician’s office to an imaging center to the operating room of a hospital. Each stop generates a record, such as doctors’ notes, test results, medical device data, discharge summaries or information pertinent to the social determinants of health, which become part of a patient’s electronic health record (EHR) in each setting.¹ To provide the highest quality patients care, these records need to be easily accessible regardless of where and when they were generated. Learn more To learn more about how you can benefit from the iConnect platform’s scalability, from simple image viewing to enterprise-level management and integration of diverse image sources and applications, visit ibm.com/ watson-health/solutions/ enterprise-imaging. How IBM iConnect Enterprise Archive can help IBM iConnect® Enterprise Archive allows you to store, manage and share all your enterprise-wide images, DICOM and non- DICOM, from disparate PACS, specialties, service lines and sites regardless of source or format. The award-winning VNA also makes it easy to extend your IHE strategy to include native XDS support. With IBM iConnect Enterprise Archive, you can access a complete view of a patient’s medical record with rich integration to the EHR, helping you to make more informed decisions for your patients. As the foundation of a holistic image management solution, IBM iConnect Enterprise Archive allows physicians to access current and historical images, as well as other essential non-DICOM patient data, using your choice of viewer. -

Predictive Analytics in Value-Based Healthcare
White paper Predictive Analytics in Value-Based Healthcare: Forecasting Risk, Utilization, and Outcomes Delivering effective value-based healthcare requires identifying Consider a model predicting the chance of a hospital and mitigating risk by anticipating and preventing adverse readmission. The input training dataset might contain events and outcomes. Predictive models have been a part of thousands of hospitalization events. Each hospital admission healthcare practice for decades. However, more advanced would include the information on a patient’s medical history, analytics have started to take shape to provide better visibility like chronic conditions and prior utilization. The dataset into characterizing a patient’s current state and future risk. would also contain the “answer” as to whether that particular With the use of big data, it is possible to build models around hospitalization resulted in a readmission within 30 days. predicting future events and outcomes, utilization, and overall Data scientists typically use specialized software (or write risk. These predictive models can be: their own) to build models that “learn” from the results of a – incorporated into a clinical workflow to facilitate care readmission to improve predictive accuracy. The model is then management and identify individuals at risk evaluated using the testing dataset, where a similar set of data – used to perform risk adjustment on quality measures is provided without the “answer” and the model’s prediction to account for patient severity is compared to the correct result (whether the patient was – employed to understand the treatment pathway with truly readmitted or not). The accuracy of a model on a testing the greatest chance of success dataset is typically what is presented as the true performance Predictive models have many potential uses, but no of the model. -

IBM Explorys Electronic Health Record (EHR) Database Data Sheet
DATA SHEET IBM Explorys Electronic Health Record (EHR) Database Confidently and efficiently generate Highlights: the data-driven insights that you – A rich, integrated, and growing living need to make business-critical clinical data set that is HIPAA- decisions enabled, including more than 63 million unique patients – A comprehensive picture of the How Users May Benefit patient experience is provided with data sourcing from integrated For life sciences organizations that require clinical delivery networks (IDNs), clinically insights, IBM® Explorys® offerings are real-world, integrated networks (CINs), and near real-time, cloud-based clinical data assets accountable care organizations and analytic solutions. They help industry (ACOs), closing the data gaps that professionals confidently and efficiently generate plague other electronic medical data-driven insights needed to make business- record (EMR)-based data sets critical decisions across a range of functional areas – Extensive data curation, quality including medical affairs, clinical development, assurance, and quality control to health economics and outcomes research, strengthen data quality, accuracy, epidemiology, and commercialization. and harmonization – Unique capabilities, including near Rich Data > Deep Insights > Confident Decisions real-time analyses, supported by strong relationships with an Built upon clinical data, captured with breadth, expanding group of providers depth, and completeness, the IBM Explorys – Turnkey functionality and advanced offerings for life sciences tap a wealth of clinical data management through seamless information for more than 63 million unique partnering with IBM's analytic tools patients. Deep, longitudinal data is available for and consulting services these patients for an average of 3-4 years. The data – Cloud-based, security-rich platform also spans the continuum of care, from ambulatory for ease-of-access and flexibility to inpatient to specialty care. -

IEEE Nanotechnology Symposium Program South Auditorium, SUNY Polytechnic Institute 257 Fuller Rd, Albany NY - 12203 Wednesday, Nov 15Th , 2017 (9:00 AM – 6:00 PM)
IEEE Nanotechnology Symposium Program South Auditorium, SUNY Polytechnic Institute 257 Fuller Rd, Albany NY - 12203 Wednesday, Nov 15th , 2017 (9:00 AM – 6:00 PM) Organizing Committee Chair Prasad Bhosale Assistant Chair (Review Committee) Devika Sil Organizing Committee Rinus Lee (IEEE EDS) Ming Yi (IEEE EDS) Alain Diebold (SUNY) Brian Walsh (IBM) Carol Boye (IBM) Cody Murray (IBM) Hao Tang (IBM) Indira Seshadri (IBM) Jingyun Zhang (IBM) Larry Clevenger (IBM) Mona Ebrish (IBM) Nicole Munro (IBM) Nicole Saulnier (IBM) Roger Quon (IBM) Mary Breton (IBM) Tenko Yamashita (IBM) Bhagawan Sahu (GLOBALFOUNDRIES) Han You (GLOBALFOUNDRIES) Kevin Ryan (GLOBALFOUNDRIES) Ralf Buengener (GLOBALFOUNDRIES) Rohit Galatage (GLOBALFOUNDRIES) Executives Alain Diebold Dean, College of Nanoscale Science & Engineering, SUNY Polytechnic Institute Fernando Guarin IEEE Fellow, EDS President Elect 2016-2017 George Gomba Vice President, Technology Research, GLOBALFOUNDRIES Mukesh Khare Vice President, Semiconductor Technology Research, IBM www.albanynanotechnology.org [email protected] Symposium Donors Keynote Address (9:15 – 10:00 AM) Fausto Bernardini VP and DE, Watson Platform for Health, IBM Research “Enabling Computational Health in the Era of Big Data” Abstract The amount and variety of data in healthcare is growing at a very rapid pace. By some estimates there are 150+ Exabytes of data in healthcare today and doubling every 24 months! In addition to the data, the amount of knowledge in medicine available in the form of publications is doubling every 18 months. The most important challenge organizations are facing is how to cope with the increasing amounts of data and knowledge and how to derive insights that matter in making decisions across the healthcare and life sciences applications. -

Delivering Artificial Intelligence in Government: Challenges and Opportunities
IBM Center for The Business of Government Delivering Artificial Intelligence in Government: Challenges and Opportunities Kevin C. Desouza Arizona State University 2018 Delivering Artificial Intelligence in Government: Challenges and Opportunities Kevin C. Desouza Arizona State University DELIVERING ARTIFICIAL INTELLIGENCE IN GOVERNMENT: CHALLENGES AND OPPORTUNITIES www.businessofgovernment.org TABLE OF CONTENTS Foreword . 4 Executive Summary . 5 Introduction . 7 Key Advances in AI . .. 7 Applying AI to Help Government . 11 Planning, Developing, and Deploying AI in the Public Sector . 14 Planning . .. 15 Development . 15 Deployment . 16 Challenges and Opportunities for Government in Implementing AI . 18 Technology and Data . 21 Challenge 1: Legacy IT Infrastructure . 21 Challenge 2: Limited IT Interoperability . 22 Challenge 3: IT Project Management Capabilities . 23 Challenge 4: Lack of Prioritization of Data-Driven Solutions . 24 Workforce . 26 Challenge 5: Public Workforce Management Practices . 26 Challenge 6: Culture of IT Ownership . 28 Challenge 7: Limited Capacity for System-Level Redesign . 30 Risk Management . .. 32 Challenge 8: Securing Systems . 32 Challenge 9: Risk Aversion . 33 Challenge 10: Ethical and Social Considerations . .. 35 Challenge 11: Governance . .. 37 Conclusion . .. 40 Appendix: Maturity Framework for AI . 41 Level 1: Unaware . 41 Priority Opportunities . 41 Level 2: Aware and Exploring . 41 Priority Opportunities . 42 Level 3: Organized and Initial System Deployments . 42 Priority Opportunities . 42 Level