The Exponential Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Appreciation of Euler's Formula
Rose-Hulman Undergraduate Mathematics Journal Volume 18 Issue 1 Article 17 An Appreciation of Euler's Formula Caleb Larson North Dakota State University Follow this and additional works at: https://scholar.rose-hulman.edu/rhumj Recommended Citation Larson, Caleb (2017) "An Appreciation of Euler's Formula," Rose-Hulman Undergraduate Mathematics Journal: Vol. 18 : Iss. 1 , Article 17. Available at: https://scholar.rose-hulman.edu/rhumj/vol18/iss1/17 Rose- Hulman Undergraduate Mathematics Journal an appreciation of euler's formula Caleb Larson a Volume 18, No. 1, Spring 2017 Sponsored by Rose-Hulman Institute of Technology Department of Mathematics Terre Haute, IN 47803 [email protected] a scholar.rose-hulman.edu/rhumj North Dakota State University Rose-Hulman Undergraduate Mathematics Journal Volume 18, No. 1, Spring 2017 an appreciation of euler's formula Caleb Larson Abstract. For many mathematicians, a certain characteristic about an area of mathematics will lure him/her to study that area further. That characteristic might be an interesting conclusion, an intricate implication, or an appreciation of the impact that the area has upon mathematics. The particular area that we will be exploring is Euler's Formula, eix = cos x + i sin x, and as a result, Euler's Identity, eiπ + 1 = 0. Throughout this paper, we will develop an appreciation for Euler's Formula as it combines the seemingly unrelated exponential functions, imaginary numbers, and trigonometric functions into a single formula. To appreciate and further understand Euler's Formula, we will give attention to the individual aspects of the formula, and develop the necessary tools to prove it. -

A Study on the Wireless Power Transfer Efficiency of Electrically Small, Perfectly Conducting Electric and Magnetic Dipoles
A study on the wireless power transfer efficiency of electrically small, perfectly conducting electric and magnetic dipoles Article Published Version Open access Moorey, C. L. and Holderbaum, W. (2017) A study on the wireless power transfer efficiency of electrically small, perfectly conducting electric and magnetic dipoles. Progress In Electromagnetics Research C, 77. pp. 111-121. ISSN 1937- 8718 doi: https://doi.org/10.2528/PIERC17062304 Available at http://centaur.reading.ac.uk/75906/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.2528/PIERC17062304 To link to this article DOI: http://dx.doi.org/10.2528/PIERC17062304 Publisher: EMW Publishing All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Progress In Electromagnetics Research C, Vol. 77, 111–121, 2017 A Study on the Wireless Power Transfer Efficiency of Electrically Small, Perfectly Conducting Electric and Magnetic Dipoles Charles L. Moorey1 and William Holderbaum2, * Abstract—This paper presents a general theoretical analysis of the Wireless Power Transfer (WPT) efficiency that exists between electrically short, Perfect Electric Conductor (PEC) electric and magnetic dipoles, with particular relevance to near-field applications. The figure of merit for the dipoles is derived in closed-form, and used to study the WPT efficiency as the criteria of interest. -

Power Waveforming: Wireless Power Transfer Beyond Time Reversal
IEEE TRANSACTIONS ON SIGNAL PROCESSING, VOL. 64, NO. 22, NOVEMBER 15, 2016 5819 Power Waveforming: Wireless Power Transfer Beyond Time Reversal Meng-Lin Ku, Member, IEEE, Yi Han, Hung-Quoc Lai, Member, IEEE, Yan Chen, Senior Member, IEEE,andK.J.RayLiu, Fellow, IEEE Abstract—This paper explores the idea of time-reversal (TR) services [1]. In particular, such an embarrassment is unavoid- technology in wireless power transfer to propose a new wireless able when wireless devices are untethered to a power grid and power transfer paradigm termed power waveforming (PW), where can only be supplied by batteries with limited capacity [2]. In a transmitter engages in delivering wireless power to an intended order to prolong the network lifetime, one immediate solution receiver by fully utilizing all the available multipaths that serve is to frequently replace batteries before the battery is exhausted, as virtual antennas. Two power transfer-oriented waveforms, en- ergy waveform and single-tone waveform, are proposed for PW but unfortunately, this strategy is inconvenient, costly and dan- power transfer systems, both of which are no longer TR in essence. gerous for some emerging wireless applications, e.g., sensor The former is designed to maximize the received power, while the networks in monitoring toxic substance. latter is a low-complexity alternative with small or even no perfor- Recently, energy harvesting has attracted a lot of attention in mance degradation. We theoretically analyze the power transfer realizing self-sustainable wireless communications with perpet- gain of the proposed power transfer system over the direct trans- ual power supplies [3], [4]. Being equipped with a rechargeable mission scheme, which can achieve about 6 dB gain, under various battery, a wireless device is solely powered by the scavenged en- channel power delay profiles and show that the PW is an ideal ergy from the natural environment such as solar, wind, motion, paradigm for wireless power transfer because of its inherent abil- vibration and radio waves. -

The Exponential Function
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln MAT Exam Expository Papers Math in the Middle Institute Partnership 5-2006 The Exponential Function Shawn A. Mousel University of Nebraska-Lincoln Follow this and additional works at: https://digitalcommons.unl.edu/mathmidexppap Part of the Science and Mathematics Education Commons Mousel, Shawn A., "The Exponential Function" (2006). MAT Exam Expository Papers. 26. https://digitalcommons.unl.edu/mathmidexppap/26 This Article is brought to you for free and open access by the Math in the Middle Institute Partnership at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in MAT Exam Expository Papers by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The Exponential Function Expository Paper Shawn A. Mousel In partial fulfillment of the requirements for the Masters of Arts in Teaching with a Specialization in the Teaching of Middle Level Mathematics in the Department of Mathematics. Jim Lewis, Advisor May 2006 Mousel – MAT Expository Paper - 1 One of the basic principles studied in mathematics is the observation of relationships between two connected quantities. A function is this connecting relationship, typically expressed in a formula that describes how one element from the domain is related to exactly one element located in the range (Lial & Miller, 1975). An exponential function is a function with the basic form f (x) = ax , where a (a fixed base that is a real, positive number) is greater than zero and not equal to 1. The exponential function is not to be confused with the polynomial functions, such as x 2. One way to recognize the difference between the two functions is by the name of the function. -
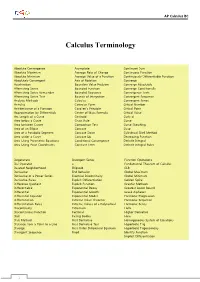
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

Using Odes to Model Drug Concentrations Within the Field of Pharmacokinetics Andrea Mcnally Augustana College, Rock Island Illinois
CORE Metadata, citation and similar papers at core.ac.uk Provided by Augustana College, Illinois: Augustana Digital Commons Augustana College Augustana Digital Commons Mathematics: Student Scholarship & Creative Mathematics Works Spring 2016 Using ODEs to Model Drug Concentrations within the Field of Pharmacokinetics Andrea McNally Augustana College, Rock Island Illinois Follow this and additional works at: http://digitalcommons.augustana.edu/mathstudent Part of the Applied Mathematics Commons Augustana Digital Commons Citation McNally, Andrea. "Using ODEs to Model Drug Concentrations within the Field of Pharmacokinetics" (2016). Mathematics: Student Scholarship & Creative Works. http://digitalcommons.augustana.edu/mathstudent/2 This Student Paper is brought to you for free and open access by the Mathematics at Augustana Digital Commons. It has been accepted for inclusion in Mathematics: Student Scholarship & Creative Works by an authorized administrator of Augustana Digital Commons. For more information, please contact [email protected]. Andrea McNally MATH-478 4/4/16 In 2009, the Food and Drug Administration discovered that individuals taking over-the- counter pain medications containing acetaminophen were at risk of unintentional overdose because these patients would supplement the painkillers with other medications containing acetaminophen. This resulted in an increase in liver failures and death over the years. To combat this problem, the FDA decided to lower the dosage from 1000 mg to 650 mg every four hours, thus reducing this risk (U.S. Food and Drug Administration). The measures taken in this example demonstrate the concepts behind the field of Pharmacology. Pharmacology is known as the study of the uses, effects, and mode of action of drugs (“Definition of Pharmacology”). -

Geometric Sequences & Exponential Functions
Algebra I GEOMETRIC SEQUENCES Study Guides Big Picture & EXPONENTIAL FUNCTIONS Both geometric sequences and exponential functions serve as a way to represent the repeated and patterned multiplication of numbers and variables. Exponential functions can be used to represent things seen in the natural world, such as population growth or compound interest in a bank. Key Terms Geometric Sequence: A sequence of numbers in which each number in the sequence is found by multiplying the previous number by a fixed amount called the common ratio. Exponential Function: A function with the form y = A ∙ bx. Geometric Sequences A geometric sequence is a type of pattern where every number in the sequence is multiplied by a certain number called the common ratio. Example: 4, 16, 64, 256, ... Find the common ratio r by dividing each term in the sequence by the term before it. So r = 4 Exponential Functions An exponential function is like a geometric sequence, except geometric sequences are discrete (can only have values at certain points, e.g. 4, 16, 64, 256, ...) and exponential functions are continuous (can take on all possible values). Exponential functions look like y = A ∙ bx, where A is the starting amount and b is like the common ratio of a geometric sequence. Graphing Exponential Functions Here are some examples of exponential functions: Exponential Growth and Decay Growth: y = A ∙ bx when b ≥ 1 Decay: y = A ∙ bx when b is between 0 and 1 your textbook and is for classroom or individual use only. your Disclaimer: this study guide was not created to replace Disclaimer: this study guide was • In exponential growth, the value of y increases (grows) as x increases. -

NOTES Reconsidering Leibniz's Analytic Solution of the Catenary
NOTES Reconsidering Leibniz's Analytic Solution of the Catenary Problem: The Letter to Rudolph von Bodenhausen of August 1691 Mike Raugh January 23, 2017 Leibniz published his solution of the catenary problem as a classical ruler-and-compass con- struction in the June 1691 issue of Acta Eruditorum, without comment about the analysis used to derive it.1 However, in a private letter to Rudolph Christian von Bodenhausen later in the same year he explained his analysis.2 Here I take up Leibniz's argument at a crucial point in the letter to show that a simple observation leads easily and much more quickly to the solution than the path followed by Leibniz. The argument up to the crucial point affords a showcase in the techniques of Leibniz's calculus, so I take advantage of the opportunity to discuss it in the Appendix. Leibniz begins by deriving a differential equation for the catenary, which in our modern orientation of an x − y coordinate system would be written as, dy n Z p = (n = dx2 + dy2); (1) dx a where (x; z) represents cartesian coordinates for a point on the catenary, n is the arc length from that point to the lowest point, the fraction on the left is a ratio of differentials, and a is a constant representing unity used throughout the derivation to maintain homogeneity.3 The equation characterizes the catenary, but to solve it n must be eliminated. 1Leibniz, Gottfried Wilhelm, \De linea in quam flexile se pondere curvat" in Die Mathematischen Zeitschriftenartikel, Chap 15, pp 115{124, (German translation and comments by Hess und Babin), Georg Olms Verlag, 2011. -

Calculus Formulas and Theorems
Formulas and Theorems for Reference I. Tbigonometric Formulas l. sin2d+c,cis2d:1 sec2d l*cot20:<:sc:20 +.I sin(-d) : -sitt0 t,rs(-//) = t r1sl/ : -tallH 7. sin(A* B) :sitrAcosB*silBcosA 8. : siri A cos B - siu B <:os,;l 9. cos(A+ B) - cos,4cos B - siuA siriB 10. cos(A- B) : cosA cosB + silrA sirrB 11. 2 sirrd t:osd 12. <'os20- coS2(i - siu20 : 2<'os2o - I - 1 - 2sin20 I 13. tan d : <.rft0 (:ost/ I 14. <:ol0 : sirrd tattH 1 15. (:OS I/ 1 16. cscd - ri" 6i /F tl r(. cos[I ^ -el : sitt d \l 18. -01 : COSA 215 216 Formulas and Theorems II. Differentiation Formulas !(r") - trr:"-1 Q,:I' ]tra-fg'+gf' gJ'-,f g' - * (i) ,l' ,I - (tt(.r))9'(.,') ,i;.[tyt.rt) l'' d, \ (sttt rrJ .* ('oqI' .7, tJ, \ . ./ stll lr dr. l('os J { 1a,,,t,:r) - .,' o.t "11'2 1(<,ot.r') - (,.(,2.r' Q:T rl , (sc'c:.r'J: sPl'.r tall 11 ,7, d, - (<:s<t.r,; - (ls(].]'(rot;.r fr("'),t -.'' ,1 - fr(u") o,'ltrc ,l ,, 1 ' tlll ri - (l.t' .f d,^ --: I -iAl'CSllLl'l t!.r' J1 - rz 1(Arcsi' r) : oT Il12 Formulas and Theorems 2I7 III. Integration Formulas 1. ,f "or:artC 2. [\0,-trrlrl *(' .t "r 3. [,' ,t.,: r^x| (' ,I 4. In' a,,: lL , ,' .l 111Q 5. In., a.r: .rhr.r' .r r (' ,l f 6. sirr.r d.r' - ( os.r'-t C ./ 7. /.,,.r' dr : sitr.i'| (' .t 8. tl:r:hr sec,rl+ C or ln Jccrsrl+ C ,f'r^rr f 9. -

Geometric and Arithmetic Postulation of the Exponential Function
J. Austral. Math. Soc. (Series A) 54 (1993), 111-127 GEOMETRIC AND ARITHMETIC POSTULATION OF THE EXPONENTIAL FUNCTION J. PILA (Received 7 June 1991) Communicated by J. H. Loxton Abstract This paper presents new proofs of some classical transcendence theorems. We use real variable methods, and hence obtain only the real variable versions of the theorems we consider: the Hermite-Lindemann theorem, the Gelfond-Schneider theorem, and the Six Exponentials theo- rem. We do not appeal to the Siegel lemma to build auxiliary functions. Instead, the proof employs certain natural determinants formed by evaluating n functions at n points (alter- nants), and two mean value theorems for alternants. The first, due to Polya, gives sufficient conditions for an alternant to be non-vanishing. The second, due to H. A. Schwarz, provides an upper bound. 1991 Mathematics subject classification (Amer. Math. Soc): 11 J 81. 1. Introduction The purpose of this paper is to give new proofs of some classical results in the transcendence theory of the exponential function. We employ some determinantal mean value theorems, and some geometrical properties of the exponential function on the real line. Thus our proofs will yield only the real valued versions of the theorems we consider. Specifically, we give proofs of (the real versions of) the six exponentials theorem, the Gelfond-Schneider theorem, and the Hermite-Lindemann the- orem. We do not use Siegel's lemma on solutions of integral linear equations. Using the data of the hypotheses, we construct certain determinants. With © 1993 Australian Mathematical Society 0263-6115/93 $A2.00 + 0.00 111 Downloaded from https://www.cambridge.org/core. -
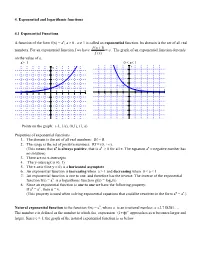
Chapter 4 Notes
4. Exponential and logarithmic functions 4.1 Exponential Functions A function of the form f(x) = ax, a > 0 , a 1 is called an exponential function. Its domain is the set of all real f (x 1) numbers. For an exponential function f we have a . The graph of an exponential function depends f (x) on the value of a. a> 1 0 < a< 1 y y 5 5 4 4 3 3 2 2 (1,a) (-1, 1/a) (-1, 1/a) 1 1 (1,a) x x -5 -4 -3 -2 -1 1 2 3 4 5 -5 -4 -3 -2 -1 1 2 3 4 5 -1 -1 -2 -2 -3 -3 -4 -4 -5 -5 Points on the graph: (-1, 1/a), (0,1), (1, a) Properties of exponential functions 1. The domain is the set of all real numbers: Df = R 2. The range is the set of positive numbers: Rf = (0, +). (This means that ax is always positive, that is ax > 0 for all x. The equation ax = negative number has no solution) 3. There are no x-intercepts 4. The y-intercept is (0, 1) 5. The x-axis (line y = 0) is a horizontal asymptote 6. An exponential function is increasing when a > 1 and decreasing when 0 < a < 1 7. An exponential function is one to one, and therefore has the inverse. The inverse of the exponential x function f(x) = a is a logarithmic function g(x) = loga(x) 8. Since an exponential function is one to one we have the following property: If au = av , then u = v. -

A Study on the Wireless Power Transfer Efficiency of Electrically
Progress In Electromagnetics Research C, Vol. 77, 111–121, 2017 A Study on the Wireless Power Transfer Efficiency of Electrically Small, Perfectly Conducting Electric and Magnetic Dipoles Charles L. Moorey1 and William Holderbaum2, * Abstract—This paper presents a general theoretical analysis of the Wireless Power Transfer (WPT) efficiency that exists between electrically short, Perfect Electric Conductor (PEC) electric and magnetic dipoles, with particular relevance to near-field applications. The figure of merit for the dipoles is derived in closed-form, and used to study the WPT efficiency as the criteria of interest. The analysis reveals novel results regarding the WPT efficiency for both sets of dipoles, and describes how electrically short perfectly conducting dipoles can achieve efficient WPT over distances that are considerably greater than their size. 1. INTRODUCTION Wireless Power Transfer holds the promise of delivering new and innovative charging solutions across a range of electronic applications, including medical implants [1, 2], consumer electronics [3, 4] and electric vehicles [5, 6]. However, the exponential decay of all electromagnetic fields with respect to increasing transfer distance means that WPT systems must be carefully designed in order to ensure that sufficient power reaches the desired end target. Mid-range WPT techniques have demonstrated that reasonably efficient WPT (≈ 40%) can be achieved over distances of the order or several metres [7, 8]. Longer transmission distances can be achieved by using electromagnetic radiation [9] (typically in the form of either microwaves or laser beam), but this is at the cost of drastic reductions in the WPT efficiency. As such, this method of WPT is typically restricted to low-power energy harvesting [10, 11] applications rather than those involving dedicated transmitters and receivers, as in mid-range systems.