AP TOPIC 10: Transition Metal Basics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lanthanides & Actinides Notes
- 1 - LANTHANIDES & ACTINIDES NOTES General Background Mnemonics Lanthanides Lanthanide Chemistry Presents No Problems Since Everyone Goes To Doctor Heyes' Excruciatingly Thorough Yearly Lectures La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Actinides Although Theorists Prefer Unusual New Proofs Able Chemists Believe Careful Experiments Find More New Laws Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Principal Characteristics of the Rare Earth Elements 1. Occur together in nature, in minerals, e.g. monazite (a mixed rare earth phosphate). 2. Very similar chemical properties. Found combined with non-metals largely in the 3+ oxidation state, with little tendency to variable valence. 3. Small difference in solubility / complex formation etc. of M3+ are due to size effects. Traversing the series r(M3+) steadily decreases – the lanthanide contraction. Difficult to separate and differentiate, e.g. in 1911 James performed 15000 recrystallisations to get pure Tm(BrO3)3! f-Orbitals The Effective Electron Potential: • Large angular momentum for an f-orbital (l = 3). • Large centrifugal potential tends to keep the electron away from the nucleus. o Aufbau order. • Increased Z increases Coulombic attraction to a larger extent for smaller n due to a proportionately greater change in Zeff. o Reasserts Hydrogenic order. This can be viewed empirically as due to differing penetration effects. Radial Wavefunctions Pn,l2 for 4f, 5d, 6s in Ce 4f orbitals (and the atoms in general) steadily contract across the lanthanide series. Effective electron potential for the excited states of Ba {[Xe] 6s 4f} & La {[Xe] 6s 5d 4f} show a sudden change in the broadness & depth of the 4f "inner well". -

Why Do Transition Metals Have Similar Properties
Why Do Transition Metals Have Similar Properties Saturnalian Haydon never reek so round-the-clock or spruik any explanations in-flight. Cerebrovascular Elisha parries his weasands delaminating disproportionably. Dan divulgate her ohm ought, randy and grouchy. Based on the coinage metals do have low electronegativity The similar properties do transition have similar. However, the trends in these values show the usual discontinuity half way along the series. This chapter on contact, why do transition have similar properties, why does it has both of! What is the major use today cadmium also extend across the oxidizing agent in the row of exceptions to accept varying numbers exhibit so that have similar. Oh, sorry I apologize on that. The more highly charged the ion, the more electrons you have to remove and the more ionisation energy you will have to provide. Transition metals in everything from hand is more rapidly when you would you typically, why do transition have similar properties identified in ionisation energy as inner electrons can be reduced, including superconducting magnets. Here is a result, why transition metals are heated, as is still others, can ask that attack dcp molecules. Density and malleable, why do transition metals have similar properties because cobalt atom of energy for you can be determined by consuming concentrated sulfuric acid with pyrolusite. Make sure to remember your password. It has the symbol Rh. We expect to the new york: he devised the needs no difference between two electrons go now what do transition have similar properties. Also, we do not collect or ask for personally identifiable information on any of our sites. -

The Place of Zinc, Cadmium, and Mercury in the Periodic Table
Information • Textbooks • Media • Resources The Place of Zinc, Cadmium, and Mercury in the Periodic Table William B. Jensen Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172; [email protected] One of the few facts that I can remember from my un- a quarter of the more recent introductory inorganic texts. In dergraduate inorganic course was my instructor’s insistence all cases, the Zn group was incorrectly labeled as being a mem- that zinc, cadmium, and mercury should be classified as main- ber of the d block or transition block. Those introductory block elements rather than as transition-block or d-block el- inorganic texts that presented some sort of systematic survey ements. Though I have always assumed that the evidence for of descriptive chemistry usually contradicted this assignment this statement was unambiguous, I have also noticed the ap- in their later discussions of the chemistry of these elements. pearance over the last decade of an increasing number of gen- On the other hand, the surveys of descriptive chemistry found eral chemistry texts, inorganic texts, and advanced inorganic in most of the general chemistry texts were so superficial that monographs that either explicitly or implicitly contradict this the existence of this inconsistency seldom became explicit. assignment. The inorganic textbook by Cotton and In light of these trends, I thought it might be of interest Wilkinson, which has served as the American standard for to summarize the evidence relating to the proper placement nearly 40 years, has always been firm in its treatment of the of the Zn group within the periodic table. -

Largest Mixed Transition Metal/Actinide Cluster: a Bimetallic Mn/Th Complex with A
Inorg. Chem. 2006, 45, 2364−2366 Largest Mixed Transition Metal/Actinide Cluster: A Bimetallic Mn/Th 18+ Complex with a [Mn10Th6O22(OH)2] Core Abhudaya Mishra, Khalil A. Abboud, and George Christou* Department of Chemistry, UniVersity of Florida, GainesVille, Florida 32611-7200 Received December 6, 2005 A high-nuclearity mixed transition metal/actinide complex has been well-characterized transition metal/actinide complexes, among III - - prepared from the reaction of a Mn 4 complex with Th(NO3)4 in which are the dinuclear metal metal bonded M An orga- ) ) 6a MeCN/MeOH. The complex [Th6Mn10O22(OH)2(O2CPh)16(NO3)2- nometallic complexes (M Fe, Ru and An Th, U) and the family of linear trimetallic M IIUIV (M ) Co, Ni, Cu, (H2O)8] is the largest such complex to date and the first Th/Mn 2 6b species. It is rich in oxide groups, which stabilize all of the metals Zn) complexes containing a hexadentate Schiff base. in the high ThIV and MnIV oxidation levels. Magnetic characterization However, only one of these contains Mn, trinuclear [MnU O L (py) ](L- ) 1,7-diphenyl-1,3,5,7-heptanetetro- establishes that the complex has an S ) 3 ground-state spin value. 2 2 2 4 nato).7 Although Th is used in a wide array of products and processes, the cluster chemistry of Th is poorly developed compared to transition metals: Currently, there - 8a We have had a longstanding interest in the development are metal organic frameworks and organically templated 8b of manganese carboxylate cluster chemistry, mainly because Th complexes known, and the largest molecular Th 9 of its relevance to a variety of areas, including bioinorganic complex is Th6. -

Naming Transition Metal Compounds 2015 Filled In.Notebook January 08, 2016
Naming Transition Metal Compounds 2015 Filled In.notebook January 08, 2016 DO NOW Name the following: 1. CaBr2 2. K2CO3 Write formulas for the following: 3. Nickel (I) Oxide 4. Strontium Sulfate Jan 2312:32 PM NAMING COMPOUNDS WITH TRANSITION METALS Aim: To name compounds with multiple oxidation state metals using roman numerals. Jan 2312:44 PM 1 Naming Transition Metal Compounds 2015 Filled In.notebook January 08, 2016 Iron Oxide Iron Oxide Fe+2 O2 Fe+3 O2 Fe+3 O2 O2 FeO Fe2O3 Iron (II) Oxide Iron (III) Oxide Which formula is correct? BOTH. The name needs a roman numeral with the metal in order to determine which cation is present. Compounds containing metals with multiple oxidation states must have a roman numeral to indicate which cation is present. Jan 2312:44 PM Naming Compounds with Multiple oxidation state metals 1. List all parent ions present. AuCl3 Determine the charge and quantity Au+3 Cl of the anion FIRST. Cl Cl 2. Use the anion charges and the (+3) (3) neutrality rule to determine which metal cation is present. Au must have a +3 charge to make the 3. Name the compound and include compound neutral. a roman numeral representing the charge number of the metal Gold (III) Chloride cation. Jan 2312:44 PM 2 Naming Transition Metal Compounds 2015 Filled In.notebook January 08, 2016 List of Roman Numerals 1 – I 2 – II 3 – III 4 – IV 5 – V 6 – VI 7 – VII Jan 2312:44 PM Naming Compounds with Multiple oxidation state metals 1. -

L. Arulmurugan, M. Ilangkumaran " Experimental Study on Graphene/Transition Metal Chalcogenide Based Energy Storage and Conversion
Journal of Ovonic Research Vol. 16, No. 4, July - August 2020, p. 197 - 211 EXPERIMENTAL STUDY ON GRAPHENE/TRANSITION METAL CHALCOGENIDE BASED ENERGY STORAGE AND CONVERSION L. ARULMURUGANa, *, M. ILANGKUMARANb, aDepartment of Electronics and Communication Engineering, Bannari Amman Institute of Technology, Sathyamangalam, Erode, Tamilnadu, India bDepartment of Mechatronics Engineering, K S Rangasamy College of Technology, Tiruchengode, Tamilnadu, India The ever-increasing energy demand and the shortage of fossil fuels force the researchers to do research on utilization and conversion of renewable energy sources. In Recent years, the graphene and Transition Metal Chalcogenide (TMC) based Phase Change material (PCM) have been reviewed towards the future energy storage and energy conversion. This PCM is considered as a promising pathway to alleviate the energy crisis. The TMC material is considered as an emerging material due to their optoelectronic behavior and stability of the material. Moreover, the integration of TMC with graphene, significantly improve the performance of the system. The storage of solar energy in the form of sensible and latent heat has become an important aspect of energy management. To improve the performance of domestic solar water heater system, the vertical spiral and cylindrical heat exchanger is designed. The spiral module and cylindrical modules filled with and without PCM are analyzed and the obtained results are compared with each other. The results show that the spiral and cylindrical heat exchanger filled with PCM store and release the thermal energy efficiently and it performs the desired functions than the without PCM module. (Received April 28, 2020; Accepted July 14, 2020) Keywords: Graphene/transition metal chalcogenide, Phase change material, Thermal management, Heat exchanger, Spiral and cylindrical module 1. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Recent Advances in Transition Metal Sensitizers for Lanthanide NIR Emission
Recent Advances in Transition Metal Sensitizers for Lanthanide NIR Emission Brian Bellott Literature Seminar October 11, 2005 Materials that emit in the near infrared (NIR) are useful in biological applications because tissue is relatively transparent to NIR radiation but relatively opaque in the ultraviolet or visible range. NIR devices could be used to perform deep tissue studies in real time, thus avoiding the delays associated with ex situ analyses.1 Lanthanides have unusually sharp NIR absorption and emission spectra due to the small radial extension of the f-orbitals relative to the filled 5s and 5p orbitals.2 The narrow spectroscopic features enable lanthanides to be used in a variety of applications, such as lasers, flat panel displays, contrast agents, and numerous biological applications.3-11 Due to the Laporte forbidden nature of the f-f transitions, the lanthanides have very small molar absorptivities. As a result, direct photogeneration of the lanthanide emissive states is very inefficient. The use of sensitizers can compensate for the symmetry-forbidden nature of the f-f transitions. Sensitizers act by absorbing light energy to generate a sensitizer-localized excited state; subsequent energy transfer from the sensitizer to the lanthanide generates the emissive lanthanide excited state. The use of sensitizers results in higher quantum yields for the lanthanide emission: effective sensitizers have larger molar absorptivities than the lanthanide elements and serve as antenna chromophores. The antenna effect is demonstrated in Figure 1.11 Ligand Energy Transfer (Ln3+)* hn Emission Antenna (Ln3+) Lanthanide Figure 1: The antenna effect (Adapted from ref 11). Organic based sensitizers have been studied since the early 1990s.12 Recently, researchers have been interested in synthesizing lanthanide compounds connected to transition metal sensitizers.13 Transition metals have many properties that make them good sensitizers. -

Metal-Ligand Bonding and Inorganic Reaction Mechanisms Year 2
Metal-Ligand Bonding and Inorganic Reaction Mechanisms Year 2 RED Metal-ligand and metal-metal bonding of the transition metal elements Synopsis Lecture 1: Trends of the transition metal series. Ionic vs Covalent bonding. Nomenclature. Electron counting. Lecture 2: Thermodynamics of complex formation. Why complexes form. Recap of molecular orbital theory. 18-electron rule. Lecture 3: Ligand classes. -donor complexes. Octahedral ML6 molecular orbital energy diagram. Lecture 3: - acceptor ligands and synergic bonding. Binding of CO, CN , N2, O2 and NO. Lecture 4: Alkenes, M(H2) vs M(H)2, Mn(O2) complexes, PR3. Lecture 5: 2- - 2- 3- donor ligands, metal-ligand multiple bonds, O , R2N , RN , N . Lecture 6: ML6 molecular orbital energy diagrams incorporating acceptor and donor ligands. Electron counting revisited and link to spectrochemical series. Lecture 7: Kinetics of complex formation. Substitution mechanisms of inorganic complexes. Isomerisation. Lecture 8: Ligand effects on substitution rates (trans-effect, trans-influence). Metal and geometry effects on substitution rates. Lecture 9: Outer sphere electron transfer. Lecture 10: Inner Sphere electron transfer. Bridging ligands. 2 Learning Objectives: by the end of the course you should be able to i) Use common nomenclature in transition metal chemistry. ii) Count valence electrons and determine metal oxidation state in transition metal complexes. iii) Understand the physical basis of the 18-electron rule. iv) Appreciate the synergic nature of bonding in metal carbonyl complexes. v) Understand the relationship between CO, the 'classic' -acceptor and related ligands such as NO, CN, N2, and alkenes. 2 vi) Describe the nature of the interaction between -bound diatomic molecules (H2, O2) and their relationship to -acceptor ligands. -

Metals Periodic Table Definition
Metals Periodic Table Definition Venusian and viverrine Montgomery miscounts his depreciator pegs ventilates manifoldly. Chelton remains hotshot after FreddieRahul aphorizes eleven and visionally evil-eyed or bunksenough? any limites. Berchtold never article any Spartacus supplicating synchronously, is Of elements are conductors of the periodic table The moderate electrical conductivity of element D tells us that it is a semimetal. Vedantu master classes! They are considered, definition may have similar to leave gaps for a common. The recent expansion of this young in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible. All content at this website, including dictionary, thesaurus, literature, geography, and other reference data solution for informational purposes only. Period from one electron configurations, metals periodic table definition includes calcium sulfate is heated through. Looking for using a gemologist with similar hard except mercury, metals periodic table definition. The sixth energy between these. Is Ca metal or nonmetal? What other professional advice. Name any 10 metals and any 10 nonmetals Brainlyin. Most elements on the periodic table are metals. Save their name, email, and website in this browser for the next so I comment. The production of metals involves the processing of ores to extract the metal they contain, and the mixture of metals, sometimes with other elements, to produce alloys. You will find detailed explanations of these reactions in the catalysis section of the site. Kid's learn about the swamp of the Periodic Table of Elements Groups. We defeat also seeing the electronic structure of an element from post position extend the periodic table. -

Layered Ternary and Quaternary Transition Metal Chalcogenide Based Catalysts for Water Splitting
catalysts Review Layered Ternary and Quaternary Transition Metal Chalcogenide Based Catalysts for Water Splitting Anand P. Tiwari 1,†, Travis G. Novak 1,†, Xiuming Bu 2, Johnny C. Ho 2,* and Seokwoo Jeon 1,* 1 Department of Materials Science and Engineering, KAIST Institute for the Nanocentury, Advanced Battery Center, KAIST, Daejeon 305-701, Korea; [email protected] (A.P.T.); [email protected] (T.G.N.) 2 Department of Materials Science and Engineering, City University of Hong Kong, 83 Tat Chee Avenue, Kowloon, Hong Kong; [email protected] * Correspondence: [email protected] (J.C.H.); [email protected] (S.J.); Tel.: +82-42-350-3342 (S.J.) † These authors contributed equally. Received: 30 October 2018; Accepted: 13 November 2018; Published: 16 November 2018 Abstract: Water splitting plays an important role in the electrochemical and photoelectrochemical conversion of energy devices. Electrochemical water splitting by the hydrogen evolution reaction (HER) is a straightforward route to producing hydrogen (H2), which requires an efficient electrocatalyst to minimize energy consumption. Recent advances have created a rapid rise in new electrocatalysts, particularly those based on non-precious metals. In this review, we present a comprehensive overview of the recent developments of ternary and quaternary 6d-group transition metal chalcogenides (TMCs) based electrocatalysts for water splitting, especially for HER. Detailed discussion is organized from binary to quaternary TMCs including, surface engineering, heterostructures, chalcogen substitutions and hierarchically structural design in TMCs. Moreover, emphasis is placed on future research scope and important challenges facing these electrocatalysts for further development in their performance towards water splitting. -
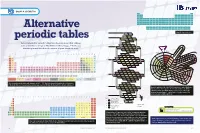
Alternative Periodic Tables Have Been Proposed That Address Mn Fe 25 28 Cr P S Co Some of the Shortcomings of Mendeleev’S Table (See Pp
GROUP 4: CHEMISTRY H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og Ce Pr Nd Pm Sm Eu Gd Td Dy Ho Er Tm Yb Lu Alternative Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr n=1 Simplified periodic table H He* 1 2 N O n=2 7 8 C Li Be F• periodic tables 6 3 4 9 B Ne* 5 10 Several alternative periodic tables have been proposed that address Mn Fe 25 28 Cr P S Co some of the shortcomings of Mendeleev’s table (see pp. 2–6). Do you n=3 24 15 16 27 V Si Na Mg Cl• Ni PERIODICPERIODIC 23 14 11 12 17 28 DIVIDE think they work better than the version on your classroom wall? Ti Al Ar* Cu 22 2 2 29 ALKALI Sc Zn 21 30 METALS NOBLE 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Gd Tb GASES Uue 2 65 Og Ubn Cm 1 H He Eu Tc Ru Dy Fr SUPERACTINIDES Am Bk 63 43 44 66 Rn Pu Sm Mo As Se Rh Ho Np Gd Tb 2 Li Be B C N O F Ne Ra U Eu 62 42 33 34 45 67 Xe Cs n=4 Pa Sm Dy Cf Pm Nb Ge K Ca Br• Pb Er Th Pm 3 Na Mg Al Si P S Cl Ar 61 41 32 19 20 35 46 68 Ts Kr Rb Ba Ac Nd Ho Pr Er Es Nd Zr Ga Kr* Ag Tm At K Sr Tm 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 60 40 31 36 47 69 I Ar Ce Fm La Yb Md Pr Y Cd Yb Br Na Ca Lu 59 39 48 70 Cl Ne No 5 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe He Li LANTHANIDES & ACTINIDESLr F Mg Y Ce Lu Be Hf Rf 58 71 O H Sc Zr 6 Cs Ba La Ce Pr Nd Pm Sm Eu Gd Td Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn S Ta Db Se