Endoscopic Ultrasonography (EUS) [For the List of Services and Procedures That Need Preauthorization, Please Refer To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endoscopic Ultrasound for the Diagnosis of Disease and Staging of Cancers in Adult Patients with Gastroenterological Or Oncological Disease: Guidelines
TITLE: Endoscopic Ultrasound for the Diagnosis of Disease and Staging of Cancers in Adult Patients with Gastroenterological or Oncological Disease: Guidelines DATE: 26 February 2014 RESEARCH QUESTION What are the evidence-based guidelines for the use of endoscopic ultrasound in the diagnosis of disease and staging of cancers in adult patient with gastroenterological or oncological disease? KEY MESSAGE Thirteen evidence-based guidelines regarding the use of endoscopic ultrasound in the diagnosis of disease and staging of cancers in adult patient with gastroenterological or oncological disease were identified. METHODS A limited literature search was conducted on key resources including PubMed, The Cochrane Library (2014, Issue 2), University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. Methodological filters were applied to limit retrieval to guidelines. The search was also limited to English language documents published between January 1, 2009 and February 11, 2014. Internet links were provided, where available. The summary of findings was prepared from the abstracts of the relevant information. Please note that data contained in abstracts may not always be an accurate reflection of the data contained within the full article. RESULTS Thirteen evidence-based guidelines regarding the staging and diagnosis of cancer and of gastrointestinal diseases were identified. Additional references of potential interest are provided in the appendix. Disclaimer: The Rapid Response Service is an information service for those involved in planning and providing health care in Canada. Rapid responses are based on a limited literature search and are not comprehensive, systematic reviews. -

Three-Way Comparative Study of Endoscopic Ultrasound-Guided
Surgical Endoscopy (2019) 33:1260–1270 and Other Interventional Techniques https://doi.org/10.1007/s00464-018-6406-7 Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: clinical outcomes and success in an International, Multicenter Study Ali Siddiqui1 · Rastislav Kunda3 · Amy Tyberg2 · Mustafa A. Arain4 · Arish Noor1 · Tayebah Mumtaz1 · Usama Iqbal1 · David E. Loren1 · Thomas E. Kowalski1 · Douglas G. Adler5 · Monica Saumoy2 · Monica Gaidhane2 · Shawn Mallery4 · Eric M. Christiansen4 · Jose Nieto6 · Michel Kahaleh2 Received: 9 November 2017 / Accepted: 24 August 2018 / Published online: 12 September 2018 © Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract Background Percutaneous cholecystostomy tube (PTGBD), endoscopic retrograde cholangiopancreatography with trans- papillary gallbladder drainage (TP), and endoscopic ultrasound-guided transmural gallbladder drainage (EGBD) using lumen-apposing metal stents (LAMS) have been offered for gallbladder decompression for acute cholecystitis in high-risk surgical patients. Yet, there are limited data comparing these therapies. Our aim was to compare the safety and efficacy of EGBD to TP and PTGBD for gallbladder drainage. Methods We retrospectively collected high-risk surgical patients from six centers with acute cholecystitis who underwent gallbladder drainage by EGBD, TP, or PTGBD. Data included technical success (gallbladder drainage), clinical success (acute cholecystitis resolution), adverse events (AE), and follow-up. Results From 2010 to 2016, 372 patients underwent gallbladder drainage, with 146 by PTGBD, 124 by TP, and 102 drained by EGBD. Technical (98% vs. 88% vs. 94%; p = 0.004) and Clinical (97% vs. -

Impact of Preoperative Endoscopic Ultrasound in Surgical Oncology
REVIEW Impact of preoperative endoscopic ultrasound in surgical oncology Endoscopic ultrasound (EUS) has a strong impact on the imaging and staging of solid tumors within or in close proximity of the upper GI tract. Technological developments during the last two decades have increased the image quality and allowed very detailed visualization of local tumor spread and lymph node affection. Current indications for EUS of the upper GI tract encompass the differentiation between benign and malignant lesions, the staging of esophageal, gastric and pancreatic cancer, and the procurement of a biopsy specimen through fine-needle aspiration. Various technical innovations during the past two decades have increased the diagnostic quality and have simultaneously strengthened the role of EUS in the clinical setting. This article will give a compressed summary on the current state of EUS and possible further technical developments. 1 KEYWORDS: 3D imaging elastosonography endoscopic ultrasound miniprobes Sascha S Chopra & oncologic surgery Michael Hünerbein† 1Department of General & Transplantation Surgery, Charité Campus Virchow-Clinic, Berlin, Conventional endoscopic ultrasound the so-called ‘miniprobes’ into the biliary system Germany Linear versus radial systems or the pancreatic duct in order to obtain high-res- †Author for correspondence: Department of Surgery & Surgical Endoscopic ultrasound (EUS) with flex- olution radial ultrasound images locally. Present Oncology, Helios Hospital Berlin, ible endoscopes is an important diagnostic and mini probes show a diameter of 2–3 mm and oper- 13122 Berlin, Germany Tel.: +49 309 417 1480 therapeutic tool, especially for the local staging ate with frequencies between 12 and 30 MHz. Fax: +49 309 417 1404 of gastrointestinal (GI) cancers, the differen- The main drawbacks of these devices are the lim- michael.huenerbein@ tiation between benign and malignant tumors, ited durability and the decreased depth of penetra- helios-kliniken.de and interventional procedures, such as biopsies tion (~2 cm). -

Cigna Medical Coverage Policies – Radiology Neck Imaging Effective November 15, 2018
Cigna Medical Coverage Policies – Radiology Neck Imaging Effective November 15, 2018 ______________________________________________________________________________________ Instructions for use The following coverage policy applies to health benefit plans administered by Cigna. Coverage policies are intended to provide guidance in interpreting certain standard Cigna benefit plans and are used by medical directors and other health care professionals in making medical necessity and other coverage determinations. Please note the terms of a customer’s particular benefit plan document may differ significantly from the standard benefit plans upon which these coverage policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a coverage policy. In the event of a conflict, a customer’s benefit plan document always supersedes the information in the coverage policy. In the absence of federal or state coverage mandates, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of: 1. The terms of the applicable benefit plan document in effect on the date of service 2. Any applicable laws and regulations 3. Any relevant collateral source materials including coverage policies 4. The specific facts of the particular situation Coverage policies relate exclusively to the administration of health benefit plans. Coverage policies are not recommendations for treatment and should never be used as treatment guidelines. This evidence-based medical coverage policy has been developed by eviCore, Inc. Some information in this coverage policy may not apply to all benefit plans administered by Cigna. These guidelines include procedures eviCore does not review for Cigna. -

Endoscopic Ultrasound: What Is It and When Should It Be Used?
CMJ0906-Willert.qxd 11/10/09 7:54 PM Page 539 ■ CLINICAL PRACTICE Clinical Medicine 2009, Vol 9, No 6: 539–43 Endoscopic ultrasound: what is it and when should it be used? Yogananda Reddy and Robert P Willert ABSTRACT – Endoscopic ultrasound (EUS) is an increasingly There are two types of commonly used echoendoscopes each available diagnostic and therapeutic tool used within the UK. with differing characteristics. Radial EUS was the first to be It has wide applications both in the gastrointestinal tract and developed and provides a 360-degree view in a plane perpendic- mediastinum with its current main uses being in the staging ular to that of the scope, similar to a computed tomography (CT) of luminal malignancies and assessment of pancreatic and image (Fig 2). Linear EUS provides a localised oblique image subepithelial lesions. The emergence of linear EUS has parallel to the scope and enables therapeutic intervention under opened up new therapeutic avenues with fine needle aspira- ultrasound (Fig 3). High frequency EUS mini-probes are also tion, trucut biopsies, coeliac plexus blocks and transmural available which can be passed down a standard biopsy channel of pseudocyst drainage all now possible. Future developments an endoscope in cases where strictures cannot be passed using a include localised brachytherapy/chemotherapy and alcohol standard EUS scope. ablation of unresectable pancreatic malignancies and EUS- guided endoscopic surgery. Indications for endoscopic ultrasound KEY WORDS: cancer staging, endoscopic ultrasound, fine The indications for EUS can be divided into several categories: needle aspiration • staging of GI malignancies • evaluating pancreaticobiliary disease Introduction • evaluating subepithelial abnormalities • evaluating extraluminal abnormalities Endoscopic ultrasound (EUS) was developed in the 1980s • staging of lung cancer but was rarely used within most of the UK. -

Endoscopic Ultrasound-Guided Placement of the Lumen-Apposing Self-Expandable Metallic Stent for Gallbladder Drainage: a Promising Technique
REVIEW ARTICLE Annals of Gastroenterology (2016) 29, 162-167 Endoscopic ultrasound-guided placement of the lumen-apposing self-expandable metallic stent for gallbladder drainage: a promising technique Rashmee Patila, Mel A. Onab, Charilaos Papafragkakisc, Sury Anandb, Sushil Duddempudib Mount Sinai Health Systems New York, USA; The Brooklyn Hospital Center Academic Affiliate of The Icahn School of Medicine at Mount Sinai Clinical Affiliate of The Mount Sinai Hospital, Brooklyn, New York; MD Anderson Cancer Center Academic and Clinical Affiliate of the University of Texas, Houston, USA Abstract Acute cholecystitis and other clinical problems requiring gallbladder removal or drainage have conventionally been treated with surgery, endoscopic retrograde cholangiopancreatography or percutaneous transhepatic drainage of the gallbladder and/or extrahepatic bile duct. Patients unable to undergo these procedures due to functional status or anatomical anomalies are candidates for endoscopic ultrasound (EUS)-guided gallbladder drainage with stent placement. The aim of this review was to evaluate the technical feasibility and efficacy of EUS-guided placement of the recently developed lumen-apposing self-expandable metallic stent (LASEMS). A literature review was performed to identify the studies describing this technique. In this review article we have summarized case series or reports describing EUS-guided LASEMS placement. The indications, techniques, limitations and complications reported are discussed. A total of 78 patients were included across all studies described thus far in the literature. Studies have reported near 100% technical and clinical success rates in selected cases. No major complications were reported. EUS-guided gallbladder drainage and LASEMS placement can be a safe and effective alternative approach in the management of selected patients. -

MRCP Vs. ERCP
MRCPMRCP vs.vs. ERCPERCP SteveSteve Harrell,Harrell, MD,MD, MSPHMSPH AdvancedAdvanced TherapeuticTherapeutic EndoscopyEndoscopy DecemberDecember 6,6, 20072007 University of Louisville InitialInitial ThoughtsThoughts ““So,So, itit isis mymy predictionprediction thatthat MRCPMRCP willwill havehave aa hugehuge effecteffect onon ERCPERCP practicepractice inin thethe UnitedUnited States.States.”” ““IfIf II hadhad aa pancreaticpancreatic oror biliarybiliary problemproblem II wouldwould searchsearch outout …… aa centercenter withwith thethe mostmost sophisticatedsophisticated noninvasivenoninvasive techniquestechniques…… veryvery quickly.quickly.”” ““WeWe allall wantwant thethe bestbest forfor ourour patients;patients; shouldshould wewe treattreat themthem differentlydifferently thanthan wewe wouldwould ourselves?ourselves?”” 5/15/985/15/98 Peter B. Cotton, MD, FRCP Medical University of South Carolina Charleston, South Carolina Universityhttp://www.ddc.musc.edu/ddc_pro/pro_development of Louisville /hot_topics/impact_MRCP-cotton.htm LearningLearning GoalsGoals KnowKnow whatwhat ERCPERCP andand MRCPMRCP standstand forfor AdvantagesAdvantages andand disadvantagesdisadvantages ofof MRCPMRCP IndicationsIndications forfor ERCPERCP PoorPoor IndicationsIndications forfor ERCPERCP ClinicalClinical UseUse inin commoncommon disordersdisorders forfor MRCPMRCP EffectsEffects ofof MRCPMRCP onon ERCPERCP inin trainingtraining CasesCases University of Louisville ERCPERCP EndoscopicEndoscopic retrograderetrograde cholangiopancreatographycholangiopancreatography -
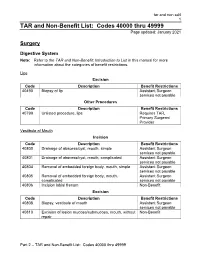
TAR and Non-Benefit List: Codes 40000 Thru 49999 Page Updated: January 2021
tar and non cd4 1 TAR and Non-Benefit List: Codes 40000 thru 49999 Page updated: January 2021 Surgery Digestive System Note: Refer to the TAR and Non-Benefit: Introduction to List in this manual for more information about the categories of benefit restrictions. Lips Excision Code Description Benefit Restrictions 40490 Biopsy of lip Assistant Surgeon services not payable Other Procedures Code Description Benefit Restrictions 40799 Unlisted procedure, lips Requires TAR, Primary Surgeon/ Provider Vestibule of Mouth Incision Code Description Benefit Restrictions 40800 Drainage of abscess/cyst, mouth, simple Assistant Surgeon services not payable 40801 Drainage of abscess/cyst, mouth, complicated Assistant Surgeon services not payable 40804 Removal of embedded foreign body, mouth, simple Assistant Surgeon services not payable 40805 Removal of embedded foreign body, mouth, Assistant Surgeon complicated services not payable 40806 Incision labial frenum Non-Benefit Excision Code Description Benefit Restrictions 40808 Biopsy, vestibule of mouth Assistant Surgeon services not payable 40810 Excision of lesion mucosa/submucosa, mouth, without Non-Benefit repair Part 2 – TAR and Non-Benefit List: Codes 40000 thru 49999 tar and non cd4 2 Page updated: January 2021 Excision (continued) Code Description Benefit Restrictions 40812 Excision of lesion mucosa/submucosa, mouth, simple Assistant Surgeon repair services not payable 40816 Excision of lesion, mouth, mucosa/submucosa, Assistant Surgeon complex services not payable 40819 Excision of frenum, labial -

Endoscopic Ultrasound Guided Fine Needle Aspiration/Biopsy
Endoscopic Ultrasound Guided Fine Needle Aspiration/Biopsy Saturday 21st October 2017 Cytology Society of the South West and South Wales Lachlan Ayres Consultant Gastroenterologist Poole Hospital NHS Foundation Trust Outline • Endoscopic ultrasound • Equipment • Cases • Poole experience EUS Equipment Linear Radial EUS Linear Radial Case 1 • 71 yr old lady • Episodic abdo pain • Normal liver function tests • US: 14mm pancreatic cyst with internal echoes. Gallstones. Otherwise normal pancreas. • Conclusion ?pseudocyst • PMH: – Crohn’s colitis – Renal cell ca (R nephrectomy 2000) – Breast ca (L mastectomy 2006) Case 1 - US Case 1 - MRCP Case 1 - MR Case 1 - EUS Stomach Duodenum Differential • Pancreatic cancer • Neuroendocrine tumour • Solitary met (RCC, breast less likely) Case 1 Pancreatic Mass Core Rapid MGG The direct smears, syringe and needle washings all show blood and its constituent cells only This is inadequate for diagnostic purposes Case 1 - Pancreatic Mass Core H&E Small fragmented cores of tissue show cellular appearances, infiltrated by atypical cells that appear crushed but also show a vacuolated clear cytoplasm. Case 1- Pancreatic Mass Core CD10 Core Vimentin These cells stain positively with CD10 and Vimentin CK7 is only focally positive. Racemase (AMACR) immunohistochemistry also shows focal cytoplasmic positivity A small focus of pancreatic neuroendocrine cells is also seen in the background Case 1 - Pancreatic Mass Core AMACR Case 1 - Pancreatic Mass Core CK7 Immunoprofile • Overall, in the context of the history of renal cell carcinoma, highly likely to represent metastatic RCC. • Further RCC specific renal markers (Pax2 and RCC) were requested • Further immunohistochemical staining with the RCC antibody show positive cytoplasmic staining and offers further support to the diagnosis of metastatic RCC. -

What Is an Endoscopic Ultrasound (EUS)?
ENDOSCOPIC ULTRASOUND (EUS) What Is an Endoscopic Ultrasound (EUS)? An endoscopic ultrasound (EUS) is a specialized procedure that blends: • Endoscopy — use of a scope to look at the inside lining of the gastrointestinal (GI) tract. • Ultrasound — use of high frequency sound waves to see detailed images of the bowel wall and nearby organs or structures. An EUS is performed by a gastroenterologist who has advanced training. The EUS scope is a thin, flexible tube with a camera and a light on the end. A tiny ultrasound probe is also attached to the end of the scope. An upper EUS looks at the upper GI system: • Walls of the upper GI tract — esophagus (the tube that links your mouth and stomach), stomach and small intestine • Nearby organs — pancreas, gallbladder and bile ducts • Nearby structures — lymph nodes, tumors, cysts and blood vessels The information provided by the AGA Institute is not medical advice and should not be considered a replacement for seeing a medical professional. July 2017 Page 1 of 2 © AGA 2017 A lower EUS looks at the lower GI system: • Bowel wall of your rectum and lower colon • Nearby organs — bladder, prostate and uterus • Nearby structures — lymph nodes and tumors • Detailed images of the anal sphincter (muscles around the anus) Fine needle aspiration (FNA): If a tissue sample is needed, your doctor will use the ultrasound image to guide a thin needle through the endoscope to take a biopsy. You won’t be able to feel this. An EUS is often performed as an outpatient procedure, like other endoscopic exams. -

About Your Endoscopic Ultrasound (EUS)
PATIENT & CAREGIVER EDUCATION About Your Endoscopic Ultrasound (EUS) This information explains your endoscopic ultrasound (EUS) at Memorial Sloan Kettering (MSK). An EUS is a procedure that uses sound waves to show images of your internal organs. It will give your doctor information about: How deep a tumor extends into your tissue. If your lymph nodes are enlarged. If there is a tumor in nearby tissues. How a tumor has responded to cancer treatment. During your EUS, your doctor may also take a sample of tissue (biopsy) with a needle. If you have cysts, your doctor may also use a needle to remove fluid to learn more information about it and understand what is making it grow. Your EUS will be done as part of another endoscopy procedure you’re having. To prepare for your EUS, review the information that your nurse gave you about the endoscopy procedure you’re having. About Your Endoscopic Ultrasound (EUS) 1/3 If you’re scheduled to have an EUS of the upper part of your digestive system, such as your esophagus (food pipe), stomach, or pancreas, follow the instructions in the resource About Your Upper Endoscopy (https://mskdirect.mskcc.org/pe/upper_endoscopy). If you’re scheduled to have an EUS of your rectum or colon (large intestine), follow the instructions in the resource that your doctor or nurse gave you, which may be: About Your Flexible Sigmoidoscopy (https://mskdirect.mskcc.org/pe/flexible_sigmoidoscopy) How to Prepare for Your Colonoscopy Using MiraLAX® (https://mskdirect.mskcc.org/pe/colonoscopy_miralax) If you have any questions about your EUS, speak with your doctor or nurse. -

Endoscopic Ultrasound (EUS) Prep Guide
Endoscopic Ultrasound (EUS) Prep Guide You have been scheduled for an Endoscopic Ultrasound (EUS). Plan ahead to help reduce your stress. Use these step-by-step instructions for a successful procedure so that your doctor can clearly view your stomach. If you have any questions, please contact the Gastroenterology office at the Guthrie Clinic at (570) 887-2852. Thank you for choosing Guthrie! Endoscopic Ultrasound (EUS) Prep Guide • Health Considerations page 2 (review today) o Medications and diagnoses o Patients on blood thinners o Diabetic patients • Get prepared page 3 (review 1-2 weeks before) o Sedation & transportation requirements • Get started page 4 (review 5 days before the procedure) • Procedure day reminders page 4 (review 5 days before & day of) • Prep Instructions* (follow the day before & day of) o Prep instructions page 4 1 Health Considerations (review today) Medications and diagnoses Please call the Gastroenterology office at the Guthrie Clinic at (570) 887-2852, select option 1 immediately if any of the following apply to you: • If you are pregnant or think you may be pregnant. • If you have had Gastric Bypass or Roux-en-Y bypass surgery. • If you need to cancel or reschedule your appointment. Patients on blood thinners If you use Aggrenox, Arixtra, Brilinta, Effient, Eliquis, Plavix, Pletal, Pradaxa, Ticlid, Xarelto, or any blood thinner (anticoagulant) or an anti-platelet drug, most patients need to stop taking these 5 days prior to procedure. • Please speak with your physician who orders this medication before stopping. If you use a blood thinner named Coumadin, Warfarin, or Jantoven you will need special instructions about stopping this drug before the procedure.