Notice: Restrictions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pennsylvania Bulletin Volume 43 (2013) Repository
Pennsylvania Bulletin Volume 43 (2013) Repository 6-29-2013 June 29, 2013 (Pages 3461-3736) Pennsylvania Legislative Reference Bureau Follow this and additional works at: https://digitalcommons.law.villanova.edu/pabulletin_2013 Recommended Citation Pennsylvania Legislative Reference Bureau, "June 29, 2013 (Pages 3461-3736)" (2013). Volume 43 (2013). 26. https://digitalcommons.law.villanova.edu/pabulletin_2013/26 This June is brought to you for free and open access by the Pennsylvania Bulletin Repository at Villanova University Charles Widger School of Law Digital Repository. It has been accepted for inclusion in Volume 43 (2013) by an authorized administrator of Villanova University Charles Widger School of Law Digital Repository. Volume 43 Number 26 Saturday, June 29, 2013 • Harrisburg, PA Pages 3461—3736 Part I Agencies in this issue See Part II page 3655 The General Assembly for the Commission on The Courts Sentencing’s Adoption of Delaware River Basin Commission Amendment to the 7th Department of Banking and Securities Department of Conservation and Natural Edition Sentencing Guidelines Resources Department of Education Department of Environmental Protection Department of General Services Department of Health Department of Public Welfare Department of Revenue Executive Board Health Care Cost Containment Council Independent Regulatory Review Commission Insurance Department Liquor Control Board Patient Safety Authority Pennsylvania Alzheimer’s Disease Planning Committee Pennsylvania Gaming Control Board Pennsylvania Public Utility Commission Philadelphia Regional Port Authority State Conservation Commission State Ethics Commission Detailed list of contents appears inside. Latest Pennsylvania Code Reporters (Master Transmittal Sheets): No. 463, June 2013 published weekly by Fry Communications, Inc. for the PENNSYLVANIA Commonwealth of Pennsylvania, Legislative Reference BULLETIN Bureau, 641 Main Capitol Building, Harrisburg, Pa. -

2018 Pennsylvania Summary of Fishing Regulations and Laws PERMITS, MULTI-YEAR LICENSES, BUTTONS
2018PENNSYLVANIA FISHING SUMMARY Summary of Fishing Regulations and Laws 2018 Fishing License BUTTON WHAT’s NeW FOR 2018 l Addition to Panfish Enhancement Waters–page 15 l Changes to Misc. Regulations–page 16 l Changes to Stocked Trout Waters–pages 22-29 www.PaBestFishing.com Multi-Year Fishing Licenses–page 5 18 Southeastern Regular Opening Day 2 TROUT OPENERS Counties March 31 AND April 14 for Trout Statewide www.GoneFishingPa.com Use the following contacts for answers to your questions or better yet, go onlinePFBC to the LOCATION PFBC S/TABLE OF CONTENTS website (www.fishandboat.com) for a wealth of information about fishing and boating. THANK YOU FOR MORE INFORMATION: for the purchase STATE HEADQUARTERS CENTRE REGION OFFICE FISHING LICENSES: 1601 Elmerton Avenue 595 East Rolling Ridge Drive Phone: (877) 707-4085 of your fishing P.O. Box 67000 Bellefonte, PA 16823 Harrisburg, PA 17106-7000 Phone: (814) 359-5110 BOAT REGISTRATION/TITLING: license! Phone: (866) 262-8734 Phone: (717) 705-7800 Hours: 8:00 a.m. – 4:00 p.m. The mission of the Pennsylvania Hours: 8:00 a.m. – 4:00 p.m. Monday through Friday PUBLICATIONS: Fish and Boat Commission is to Monday through Friday BOATING SAFETY Phone: (717) 705-7835 protect, conserve, and enhance the PFBC WEBSITE: Commonwealth’s aquatic resources EDUCATION COURSES FOLLOW US: www.fishandboat.com Phone: (888) 723-4741 and provide fishing and boating www.fishandboat.com/socialmedia opportunities. REGION OFFICES: LAW ENFORCEMENT/EDUCATION Contents Contact Law Enforcement for information about regulations and fishing and boating opportunities. Contact Education for information about fishing and boating programs and boating safety education. -

Bradford County
BRADFORD COUNTY START BRIDGE SD MILES PROGRAM IMPROVEMENT TYPE TITLE DESCRIPTION COST PERIOD COUNT COUNT IMPROVED Bridge superstructure rehabilitation on PA 514 over the North Branch of Towanda BASE Bridge Rehabilitation PA 514 over the North Branch of Towanda Creek Creek in Granville Township 1 $ 1,000,000 1 0 0 BASE Bridge Replacement PA 414 over Towanda Creek Bridge replacement on PA 414 over Towanda Creek in Franklin Township 1 $ 2,000,000 1 1 0 Bridge replacement on State Route 1026 over Tributary to Wyalusing Creek in Pike BASE Bridge Replacement State Route 1026 over Tributary to Wyalusing Creek Township 1 $ 140,000 1 1 0 BASE Bridge Replacement PA 367 over Steam Mill Creek Bridge replacement on PA 367 over Steam Mill Creek in Tuscarora Township 2 $ 740,000 1 1 0 Bridge superstructure rehabilitation on PA 367 over Fargo Creek in Tuscarora BASE Bridge Rehabilitation PA 367 over Fargo Creek Township 3 $ 1,120,000 1 1 0 Township Road 402 over the South Branch of Towanda Bridge replacement on Township Road 402 over the South Branch of Towanda BASE Bridge Replacement Creek Creek in Monroe Township 1 $ 1,050,000 1 1 0 Bridge replacement on Township Road 623 over Tomjack Creek in Smithfield BASE Bridge Replacement Township Road 623 over Tomjack Creek Township 1 $ 600,000 1 1 0 Bridge replacement on US 6 over Sugar Creek in Columbia Township and a Bridge Superstructure Rehabilitation on US 6 over Sugar Creek in West Burlington BASE Bridge Replacement US 6 over Sugar Creek Township 1 $ 1,134,000 2 2 0 BASE Bridge Replacement State Route -

Wild Trout Waters (Natural Reproduction) - September 2021
Pennsylvania Wild Trout Waters (Natural Reproduction) - September 2021 Length County of Mouth Water Trib To Wild Trout Limits Lower Limit Lat Lower Limit Lon (miles) Adams Birch Run Long Pine Run Reservoir Headwaters to Mouth 39.950279 -77.444443 3.82 Adams Hayes Run East Branch Antietam Creek Headwaters to Mouth 39.815808 -77.458243 2.18 Adams Hosack Run Conococheague Creek Headwaters to Mouth 39.914780 -77.467522 2.90 Adams Knob Run Birch Run Headwaters to Mouth 39.950970 -77.444183 1.82 Adams Latimore Creek Bermudian Creek Headwaters to Mouth 40.003613 -77.061386 7.00 Adams Little Marsh Creek Marsh Creek Headwaters dnst to T-315 39.842220 -77.372780 3.80 Adams Long Pine Run Conococheague Creek Headwaters to Long Pine Run Reservoir 39.942501 -77.455559 2.13 Adams Marsh Creek Out of State Headwaters dnst to SR0030 39.853802 -77.288300 11.12 Adams McDowells Run Carbaugh Run Headwaters to Mouth 39.876610 -77.448990 1.03 Adams Opossum Creek Conewago Creek Headwaters to Mouth 39.931667 -77.185555 12.10 Adams Stillhouse Run Conococheague Creek Headwaters to Mouth 39.915470 -77.467575 1.28 Adams Toms Creek Out of State Headwaters to Miney Branch 39.736532 -77.369041 8.95 Adams UNT to Little Marsh Creek (RM 4.86) Little Marsh Creek Headwaters to Orchard Road 39.876125 -77.384117 1.31 Allegheny Allegheny River Ohio River Headwater dnst to conf Reed Run 41.751389 -78.107498 21.80 Allegheny Kilbuck Run Ohio River Headwaters to UNT at RM 1.25 40.516388 -80.131668 5.17 Allegheny Little Sewickley Creek Ohio River Headwaters to Mouth 40.554253 -80.206802 -
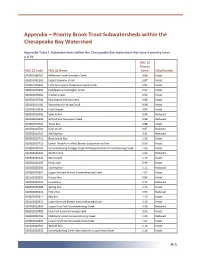
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

FFY 2009 Northern Tier TIP Highway & Bridge
FFY 2009 Northern Tier TIP Highway & Bridge Original US DOT Approval Date: 10/01/2008 Current Date: 06/30/2010 Bradford MPMS #: 65102 Municipality: Title: RR Line Item NorthernTier Route: Section: A/Q Status: Improvement Type: RR High Type Crossing Est. Let Date: Actual Let Date: Geographic Limits: Northern Tier Region of District 3Miscellaneous Projects Narrative: (Tioga, Sullivan, and Bradford Counties) - Miscellaneous Projects - Rail Crossing Improvements TIP Program Years ($000) Phase Fund FY 2009 FY 2010 FY 2011 FY 2012 2nd 4 Years 3rd 4 Years CONRRX $43 $2 $46 $48 $0 $0 $43 $2 $46 $48 $0 $0 Total FY 2009-2012 Cost $139 MPMS #: 71069 Municipality: Title: Enhancment Line Item Route: Section: A/Q Status: Improvement Type: Transportation Enhancement Est. Let Date: Actual Let Date: Geographic Limits: Northern Tier RegionEnhancement Line Item Narrative: (Bradford, Sullivan and Tioga Counties) - Transportation Enhancement Line Item TIP Program Years ($000) Phase Fund FY 2009 FY 2010 FY 2011 FY 2012 2nd 4 Years 3rd 4 Years CONSTE $435 $102 $470 $489 $0 $0 $435 $102 $470 $489 $0 $0 Total FY 2009-2012 Cost $1,496 MPMS #: 85224 Municipality: Title: Endless Mt. Facility Route: Section: A/Q Status: Improvement Type: Miscellaneous Est. Let Date: Actual Let Date: Geographic Limits: Endless Mountains. Narrative: Public Transit Facility-Flex TIP Program Years ($000) Phase Fund FY 2009 FY 2010 FY 2011 FY 2012 2nd 4 Years 3rd 4 Years CONSTP $65 $0 $0 $0 $0 $0 CONGEN $16 $0 $0 $0 $0 $0 $81 $0 $0 $0 $0 $0 Total FY 2009-2012 Cost $81 Page 1 of 152 FFY 2009 Northern Tier TIP Highway & Bridge Original US DOT Approval Date: 10/01/2008 Current Date: 06/30/2010 Bradford MPMS #: 86604 Municipality: Title: Group 3-08-RPM Route: Section: A/Q Status: Improvement Type: Reflective Pavement Markers Est. -

Notice Classification of Wild Trout Streams Proposed Additions to List April 2013
Notice Classification of Wild Trout Streams Proposed Additions to List April 2013 Under 58 Pa. Code §57.11 (relating to listing of wild trout streams), it is the policy of the Fish and Boat Commission (Commission) to accurately identify and classify stream sections supporting naturally reproducing populations of trout as wild trout streams. The Commission’s Fisheries Management Division maintains the list of wild trout streams. The Executive Director, with the approval of the Commission, will from time-to-time publish the list of wild trout streams in the Pennsylvania Bulletin. The listing of a stream section as a wild trout stream is a biological designation that does not determine how it is managed. The Commission relies upon many factors in determining the appropriate management of streams. At the next Commission meeting on April 15 and 16, 2013, the Commission will consider changes to its list of wild trout streams. Specifically, the Commission will consider the addition of the following streams or portions of streams to the list: County Stream Name Tributary To Section Limits Bedford Big Break Hollow Wallacks Branch Headwaters to mouth Bedford Ciana Run Rhodes Run Headwaters to mouth Bedford Deep Hollow Run Pavia Run Headwaters to mouth Headwaters to the confluence with Deep Bedford Pavia Run Bobs Creek Hollow Run Raystown Branch Bedford Sugar Camp Run Juniata River Headwaters to mouth Blair Diamond Run Bobs Creek Headwaters to mouth Bucks Cuttalossa Creek Delaware River Headwaters to mouth Carbon Fireline Creek Lehigh River Headwaters -

Pennsylvania Wild Trout Waters (Natural Reproduction) - November 2018
Pennsylvania Wild Trout Waters (Natural Reproduction) - November 2018 Length County of Mouth Water Trib To Wild Trout Limits Lower Limit Lat Lower Limit Lon (miles) Adams Birch Run Long Pine Run Reservoir Headwaters dnst to mouth 39.950279 -77.444443 3.82 Adams Hosack Run Conococheague Creek Headwaters dnst to mouth 39.914780 -77.467522 2.90 Adams Latimore Creek Bermudian Creek Headwaters dnst to mouth 40.003613 -77.061386 7.00 Adams Little Marsh Creek Marsh Creek Headwaters dnst to T-315 39.842220 -77.372780 3.80 Adams Marsh Creek Out of State Headwaters dnst to SR0030 39.853802 -77.288300 11.12 Adams Opossum Creek Conewago Creek Headwaters dnst to mouth 39.931667 -77.185555 12.10 Adams Stillhouse Run Conococheague Creek Headwaters dnst to mouth 39.915470 -77.467575 1.28 Allegheny Allegheny River Ohio River Headwater dnst to conf Reed Run 41.751389 -78.107498 21.80 Allegheny Kilbuck Run Ohio River Headwaters to UNT at RM 1.25 40.516388 -80.131668 5.17 Allegheny Little Sewickley Creek Ohio River Headwaters dnst to mouth 40.554253 -80.206802 7.91 Armstrong Birch Run Allegheny River Headwaters dnst to mouth 41.033300 -79.619414 1.10 Armstrong Bullock Run North Fork Pine Creek Headwaters dnst to mouth 40.879723 -79.441391 1.81 Armstrong Cornplanter Run Buffalo Creek Headwaters dnst to mouth 40.754444 -79.671944 1.76 Armstrong Cove Run Sugar Creek Headwaters dnst to mouth 40.987652 -79.634421 2.59 Armstrong Crooked Creek Allegheny River Headwaters to conf Pine Rn 40.722221 -79.102501 8.18 Armstrong Foundry Run Mahoning Creek Lake Headwaters -

Loyalsock Creek Watershed Presented By: Jim Rogers, Theresa Black, Don Muraco, and Todd Hillegas Overviewoverview Ofof Thethe Projectproject
Loyalsock Creek Watershed Presented by: Jim Rogers, Theresa Black, Don Muraco, and Todd Hillegas OverviewOverview ofof thethe ProjectProject •• PurposePurpose •• HowHow sitessites werewere evaluatedevaluated •• WhatWhat waswas foundfound •• TributariesTributaries •• ConclusionConclusion •• AcknowledgementsAcknowledgements PurposePurpose •• TheThe studystudy waswas toto identifyidentify andand documentdocument thethe overalloverall physicalphysical healthhealth ofof thethe LoyalsockLoyalsock CreekCreek WatershedWatershed andand itsits majormajor tributariestributaries suchsuch as:as: – Elk Creek – Hoagland branch of Elk Creek – Little Loyalsock – Lopez Creek – Mill Creek (Montoursville) – Mill Creek (Warrensville) – Ogdonia Creek – Plunketts Creek – Wallis Run PhysicalPhysical AssessmentAssessment •• Identify:Identify: –– ErosionErosion concernsconcerns –– LandLand andand gravelgravel barsbars –– LocationsLocations ofof bridges,bridges, roadsroads andand damsdams –– RiparianRiparian evaluationevaluation –– InvasiveInvasive plantplant speciesspecies identificationidentification SiteSite EvaluationEvaluation •• HighHigh bankbank angleangle •• HighHigh bankbank heightheight •• HighHigh rootroot densitydensity •• HighHigh particleparticle sizesize SiteSite EvaluationEvaluation •• ModerateModerate bankbank heightheight •• ModerateModerate bankbank angleangle •• HighHigh densitydensity ofof rootsroots •• ModerateModerate particleparticle sizesize SiteSite EvaluationEvaluation •• LowLow bankbank heightheight •• HighHigh bankbank angleangle •• -

Fishing Summary Fishing Summary
2019PENNSYLVANIA FISHING SUMMARY Summary of Fishing Regulations and Laws MENTORED YOUTH TROUT DAYS March 23 (regional) and April 6 (statewide) WHAT’S NEW FOR 2019 l Changes to Susquehanna and Juniata Bass Regulations–page 11 www.PaBestFishing.com l Addition and Removal to Panfish Enhancement Waters–page 15 PFBC social media and mobile app: l Addition to Catch and Release Lakes Waters–page 15 www.fishandboat.com/socialmedia l Addition to Misc. Special Regulations–page 16 Multi-Year Fishing Licenses–page 5 18 Southeastern Regular Opening Day 2 TROUT OPENERS Counties March 30 AND April 13 for Trout Statewide www.GoneFishingPa.com Go Fishin’ in Franklin County Chambersburg Trout Derby May 4-5, 2019 Area’s #1 Trout Derby ExploreFranklinCountyPA.com Facebook.com/FCVBen | Twitter.com/FCVB 866-646-8060 | 717-552-2977 2 www.fishandboat.com 2019 Pennsylvania Fishing Summary Use the following contacts for answers to your questions or better yet, go onlinePFBC to the PFBC LOCATIONS/TABLE OF CONTENTS website (www.fishandboat.com) for a wealth of information about fishing and boating. FOR MORE INFORMATION: THANK YOU STATE HEADQUARTERS CENTRE REGION OFFICE FISHING LICENSES: for the purchase 1601 Elmerton Avenue 595 East Rolling Ridge Drive Phone: (877) 707-4085 of your fishing P.O. Box 67000 Bellefonte, PA 16823 Harrisburg, PA 17106-7000 Phone: (814) 359-5110 BOAT REGISTRATION/TITLING: Phone: (866) 262-8734 license! Phone: (717) 705-7800 Hours: 8:00 a.m. – 4:00 p.m. The mission of the Pennsylvania Hours: 8:00 a.m. – 4:00 p.m. Monday through Friday PUBLICATIONS: Fish & Boat Commission is to Monday through Friday BOATING SAFETY Phone: (717) 705-7835 protect, conserve, and enhance the PFBC WEBSITE: EDUCATION COURSES Commonwealth’s aquatic resources, www.fishandboat.com Phone: (888) 723-4741 and provide fishing and boating www.fishandboat.com/socialmedia opportunities. -

Notice Classification of Wild Trout Streams Proposed Additions And
Notice Classification of Wild Trout Streams Proposed Additions and Revisions July 2018 Under 58 Pa. Code §57.11 (relating to listing of wild trout streams), it is the policy of the Fish and Boat Commission (Commission) to accurately identify and classify stream sections supporting naturally reproducing populations of trout as wild trout streams. The Commission’s Fisheries Management Division maintains the list of wild trout streams. The Executive Director, with the approval of the Commission, will from time-to-time publish the list of wild trout streams in the Pennsylvania Bulletin. The listing of a stream section as a wild trout stream is a biological designation that does not determine how it is managed. The Commission relies upon many factors in determining the appropriate management of streams. At the next Commission meeting on July 9 and 10, 2018, the Commission will consider changes to its list of wild trout streams. Specifically, the Commission will consider the addition of the following streams or portions of streams to the list: County of Mouth Stream Name Section Limits Tributary To Mouth Lat/Lon Little Brubaker Headwaters to UNT 40.706651 Cambria Brubaker Run Run at RM 1.54 78.694125 40.713405 Cambria Rock Run Headwaters to Mouth Chest Creek 78.681093 UNT to 40.713489 Cambria Beaverdam Run Headwaters to Mouth Beaverdam Run 78.533421 (RM 0.56) 40.680921 Cambria Wyerough Run Headwaters to Mouth Glendale Lake 78.582846 41.482460 Cameron Hart Run Headwaters to Mouth West Creek 78.317917 Horning Hollow 41.477402 Cameron Headwaters -

2021-02-02 010515__2021 Stocking Schedule All.Pdf
Pennsylvania Fish and Boat Commission 2021 Trout Stocking Schedule (as of 2/1/2021, visit fishandboat.com/stocking for changes) County Water Sec Stocking Date BRK BRO RB GD Meeting Place Mtg Time Upper Limit Lower Limit Adams Bermudian Creek 2 4/6/2021 X X Fairfield PO - SR 116 10:00 CRANBERRY ROAD BRIDGE (SR1014) Wierman's Mill Road Bridge (SR 1009) Adams Bermudian Creek 2 3/15/2021 X X X York Springs Fire Company Community Center 10:00 CRANBERRY ROAD BRIDGE (SR1014) Wierman's Mill Road Bridge (SR 1009) Adams Bermudian Creek 4 3/15/2021 X X York Springs Fire Company Community Center 10:00 GREENBRIAR ROAD BRIDGE (T-619) SR 94 BRIDGE (SR0094) Adams Conewago Creek 3 4/22/2021 X X Adams Co. National Bank-Arendtsville 10:00 SR0234 BRDG AT ARENDTSVILLE 200 M DNS RUSSELL TAVERN RD BRDG (T-340) Adams Conewago Creek 3 2/27/2021 X X X Adams Co. National Bank-Arendtsville 10:00 SR0234 BRDG AT ARENDTSVILLE 200 M DNS RUSSELL TAVERN RD BRDG (T-340) Adams Conewago Creek 4 4/22/2021 X X X Adams Co. National Bank-Arendtsville 10:00 200 M DNS RUSSEL TAVERN RD BRDG (T-340) RT 34 BRDG (SR0034) Adams Conewago Creek 4 10/6/2021 X X Letterkenny Reservoir 10:00 200 M DNS RUSSEL TAVERN RD BRDG (T-340) RT 34 BRDG (SR0034) Adams Conewago Creek 4 2/27/2021 X X X Adams Co. National Bank-Arendtsville 10:00 200 M DNS RUSSEL TAVERN RD BRDG (T-340) RT 34 BRDG (SR0034) Adams Conewago Creek 5 4/22/2021 X X Adams Co.